In my presentation before the FDA at yesterday’s public hearing, I made some specific suggestions, including the use of an FDA-designated hash tag to be included in each branded Tweet posted by pharmaceutical companies. If each product was assigned a unique hash tag by the FDA and all product tweets were required to include that hash tag, then the FDA, consumers, and healthcare professionals could easily review all the product tweets and ensure they obey regulations regarding fair balance presentation.
After my presentation, Tom Abrams, director of FDA’s Division of Drug Marketing, Advertising, and Communications (DDMAC), thanked me for presenting specific solutions that the FDA could consider when it creates new guidelines for the promotion of FDA-regulated products on the Internet and social media sites.
As opposed to the first Internet FDA public hearing in 1996, this one hammered into the FDA’s head how important the Internet is for health information seekers. Speaker after speaker made the point: the Internet can no longer be ignored if you are serious about protecting the public health. This time, pharmaceutical companies also made the same point.
In 1996, only visionaries could imagine how important the Internet would be in the health arena. FDA is not visionary, so the agency can be excused for not acting in 1996. This time, they have seen the light and have even used the Internet themselves to help improve public health.
I got a sense of urgency from the pharmaceutical company presenters. The industry is worried about the vast amount of user-generated health information and resources on the Internet. The industry’s share of voice on the Internet — especially the social media part of the Internet — is rapidly be dwarfed. Drug companies worry about that and they see that they need to get into the conversation. Guidelines will help them do that.
Google suggested a new way to present Rx branded paid search ads — you can read about that on EyeOnFDA here. I think the FDA will sanction this idea in its first-ever Internet specific guidelines.
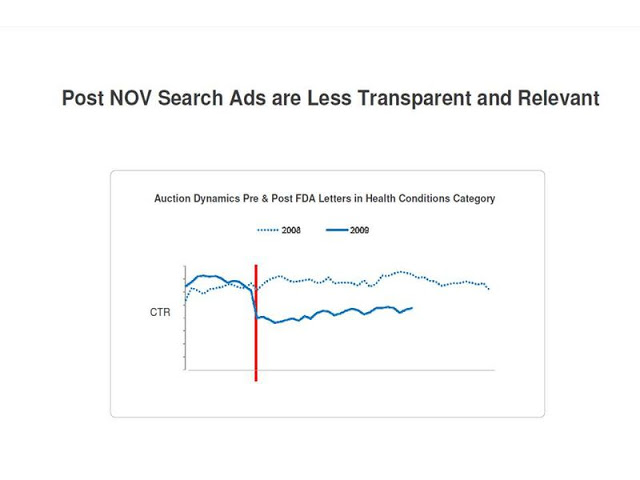
Google presented this chart, which dramatically illustrates the effect of FDA’s 14 notice of violation letters on clickthrough rates of ads that did not include the product name in the ad or the URL:
More than anything, this kind of data impresses the FDA. I am sure it shook them up yesterday.
Google, however, did not mention sidewiki. Let me repeat/paraphrase what I said in my presentation yesterday:








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




