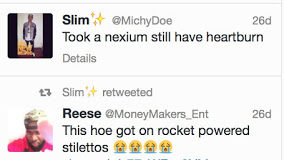
FDA has just released a new draft guidance document: “Presenting Risk Information in Prescription Drug and Medical Device Promotion” (find the pdf file here).
I haven’t read the guidance yet, but according to a Reuters report, the guidance has some specific to TV ads:
“Television ads for drugs and medical devices should avoid distracting images and music that can reduce viewers’ comprehension of potential side effects, U.S. regulators advised in guidelines proposed on Tuesday.
“Advertisements also should use similar type styles and voice-overs when conveying benefits and risks, the Food and Drug Administration said.”
It seems to me that contrary to public statements by FDA officials and bloggers (eg, “FDA to Online Marketers: Same Rules Apply“), the same rules do NOT apply to all media.
Obviously, voice-overs and moving images, which are a topic of this new guidance, are not found in print ads. If specific guidance is required about TV ads to address that medium’s unique messaging traits, then surely specific guidance for use of social media and the Internet that addresses THAT medium’s specific traits is also appropriate. Maybe not necessary, though, if you wish to keep your head in the sand and hope that the FDA won’t send you a letter.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)