A comScore study found that “sponsored link exposures to U.S. Internet users declined more than 50 percent immediately after … FDA warning letters were issued to pharmaceutical manufacturers concerning the exclusion of fair balance language in sponsored link advertising” (see “FDA Warning Letters Caused Dramatic Decline in Sponsored Link Exposures“).
I plotted the data in the following chart:
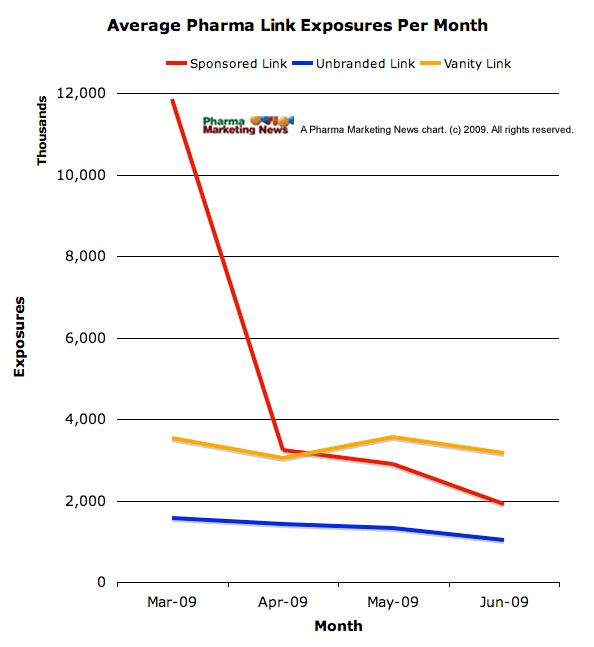
For more background on the FDA letters, see “FDA’s Actions Speak Louder than Its Words: On the Internet It’s the Medium as Well as the Message!“
Apparently, the pharmaceutical industry is now FDA Regulation-shy with regard to branded search ads, even though Google offered the industry a “redirected URL” work-around (see “St. Google Slays the FDA Dragon?“).
I wonder if pharma marketers agree with me that the FDA may take a look at the use of redirected URLs in search ads (see “The Next FDA Concern May be the Use of ‘Redirect’ URLs“) and have decided to wait (or demand) some more specific guidance on this and other Internet regulatory issues?









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




