The Food and Drug Administration Amendments Act (FDAAA), passed by Congress in 2007, authorized the FDA to require postmarketing studies for a prescription drug’s approval. Many drug approvals were fast-tracked on the promise that pharma companies would meet study deadlines and supply the FDA with data about any adverse events discovered via observational postmarketing studies after market approval.
So, how well has the FDA held pharma companies feet to the fire regarding postmarketing commitments under FDAAA jurisdiction?
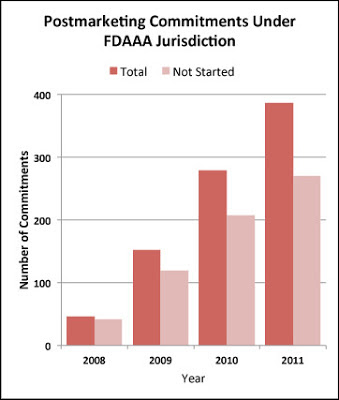
Not very well at all, according a Research Letter published in the July 10, 2013, issue of JAMA. The authors of the study found that NONE (zero) of the 865 studies under FDAAA jurisdiction from 2008 through 2011 have been completed. Of the 387 studies mandated in 2011, 271 (70%) have not even begun (i.e., are “pending” in FDA jargon; see chart below).
“For those newer studies required under theFDAAA, which are steadily increasing each year, theFDA must enforce the law
against companies failing to comply with study requirements,” say the researchers. “Under the FDAAA, the FDA can issue warning letters and initiate litigation for significant failures, including seizures and injunctions. These regulatory actions can help ensure the timely conduct and submission of adequate studies, which will ultimately strengthen the FDA’s ongoing monitoring of prescription drug safety.”
The likelihood of the FDA initiating “litigation,” “seizures,” or “injunctions” is practically nil, IMHO. What do you think?








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)