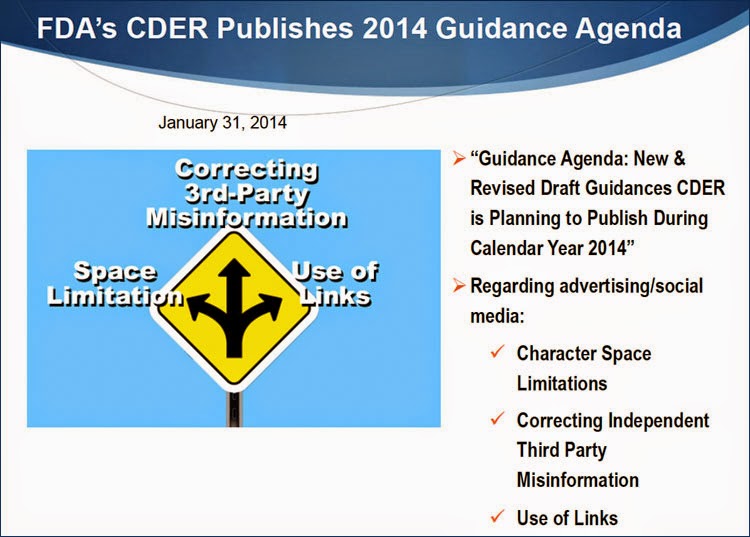
In January, 2014, FDA published its “Guidance Agenda: New & Revised Draft Guidances CDER is Planning to Publish During Calendar Year 2014,” which included these promised social media/Internet guidances under the “Advertising” category:
- Internet/Social Media Platforms with Character Space Limitations: Presenting Risk and Benefit Information for Prescription Drugs and Medical Devices
- Internet/Social Media Platforms: Correcting Independent-Third Party Misinformation About Prescription Drugs and Medical Devices
- Internet/Social Media Advertising and Promotional Labeling of Prescription Drugs and Medical Devices – Use of Links
#1 and #2 were just published (see “FDA to Pharma: Forget About Tweets! But You Can Host Discussion Forums About Branded Rx Products, Sorta, & Kinda Correct Misinformation“).
Will FDA publish Guidance #3 before the July 9, 2014, deadline imposed by Congress (see here)? Or will it miss that deadline? When it does publish this guidance, will it address ALL the issues about links raised by the FDA prior to the November, 2009, public hearing? What’s the opinion of pharma marketers regarding these issues? Read on.
Links
Prior to the November, 2009, public hearing, FDA posted these questions and requests for clarification about links:
- The agency is interested in any comments about the appropriateness of various techniques regarding the use of links (including between various social media tools) and data or research about whether or not users find these approaches to be misleading.
- Should parameters be established for links to and from Web sites?
- The agency is interested in any data or research concerning the frequency with which users actually click on different categories of links (e.g., banner ads, links within Web sites, sponsored links, organic search result links) to get additional information about products.
An example of the kind of link for which FDA may provide guidance:
Pharmaceutical Company X makes drug B that cures Y. The site has links to Y support group. On the Y support group one member writes “did you know drug B also works to cure Z. That’s what members in a support group do they talk openly. Is Company X sell off label? No. Is company X responsible? No. Can the member get the drug to cure Z? Maybe. From a doctor? Not without consent. Should the links be allowed yes.
Links were discussed by several presenters at the public hearing. I also surveyed Pharma Marketing News/Blog readers to get their opinions. The survey was closed on February 26, 2010 after collecting responses from 274 people. A summary of the results — including 731 comments — was submitted to the FDA. You can download the results here (pdf).
When asked “When is the use of links appropriate? Should parameters be established for links to and from Web sites?,” 55% of 181 survey respondents said “Yes” whereas only 24% said “No” (see chart):
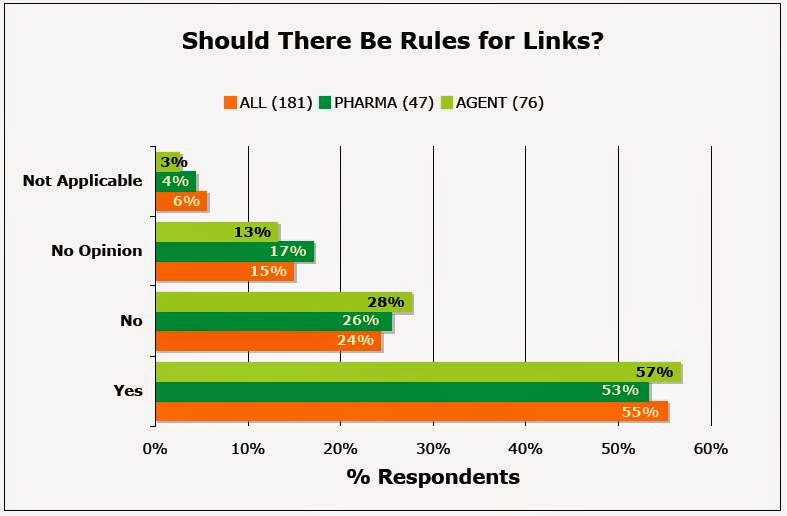 |
| PHARMA=pharma industry respondents, AGENT=pharma ad/marketing agency respondents |
The comments were more interesting:
Probably, but good luck drafting them.
Not practical to police this. it is a hyperlinked world.
If a sponsor is going to itself enable or allow a link to another site where information
about that sponsors product is known to be posted and or discussed then the sponsor is
responsible for monitoring the information from an accuracy, appriateness (sic) and legal
point of view using best “commercially reasonable” efforts. If information is posted that
is incorrect, inapropriate or illegal then it is the sponsors responsibility to take all
necessary steps to correct, change, eliminate or comment about the offending
information to the site owners where the information is posted.
A simple notice that the user is being redirected to a new site should suffice. Most
audiences are sophisticated these days to know how these campaigns work and if they
don’t want to continue on to read about a treatment they don’t have to.
blogs are often used to draw traffic to a website to restrict this handcuffs companies and
also robs the public from accessing the support documents for the view they are
reading. Even FDA has links.
Linking of off-label information from an unbranded site is clearly a no-no. I am
surprised companies would even try that trick.
I anxiously await FDA’s 3rd and final Internet/SM guidance document it promised for 2014 and hope it addresses the above comments.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




