On April 1, 2009, my “April Fool’s” joke press release announcing that the FDA issued draft guidance for use of social media for Rx drug marketing created quite a stir among pharmaceutical industry pundits and executives (see “FDA Issues First-ever Draft Guidance on Pharma’s Use of Social Media!“).
Later that month, I drafted a petition calling upon the FDA to convene a public hearing on the issue. The petition stated:
“…it is necessary for the FDA to convene a PUBLIC HEARING where ALL stakeholders can participate in a discussion of the issues and provide the FDA with a good understanding of the Internet as it exists today and tomorrow. That understanding and knowledge can then be the basis for issuing guidance that will allow the pharmaceutical industry to contribute to the conversation online” (see “FDA, Tear Down This Wall! A Draft Petition Calling for a Public Hearing“).
Well, the FDA has now actually submitted a notice to the Federal Register (to be published Monday, September 21, 2009) calling for a public hearing on the Promotion of Food and Drug Administration-Regulated Medical Products Using the Internet and Social Media Tools:
Docket No. FDA-2009-N-0441, CDER 200994. Promotion of Food and Drug Administration-Regulated Medical Products Using the Internet and Social Media Tools; Notice of Public Hearing (Notice of public hearing; request for comments). [OFR PRE-PUB: PDF of notice] Public Hearing on November 12, 2009 And November 13, 2009 Comments due February 28, 2010
[FIND THE PDF VERSION OF THE NOTICE ATTACHED TO THIS POST.]
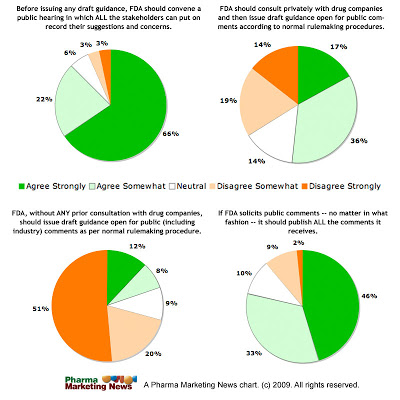
The pharmaceutical industry and readers of this blog have long supported such a hearing. See this Pharma Marketing News article:
“Developing Guidelines for Pharma’s Use of the Internet & Social Media” (FREE!) to see the results of the survey “Should FDA Convene a Public Hearing on Use of Social Media by Pharma?“








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




