As reported in today’s AdAge, “Bayer Healthcare Pharmaceuticals is pulling a 60-second ad for birth-control pill Yaz. The move comes after the U.S. Food and Drug Administration expressed concerns that two ads for the drug go too far in suggesting the drug could help overcome PMS and acne” (see “Bayer to Pull Yaz Ad After FDA Warning“).
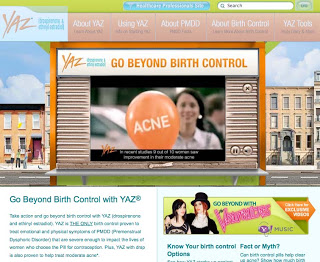
The FDA criticized the ad, ‘Balloons,’ which is still in rotation and can be seen on the YAZ product website (see image above; click for enlarged view). “The warning letter said that while Yaz had been approved as a contraceptive and a treatment for PMDD (premenstrual dysphoric disorder) and in some cases for acne, the TV ads could be viewed as suggesting the drug also remedied PMS and broader conditions of acne.”
This broadcast DTC (direct-to-consumer) ad has been around for quite some time and I’m sure everyone that Bayer wished to reach by the ad has seen it at least once, maybe several times. So, this warning letter is just another example of the FDA closing the barn door after the cow has left (see, for example, “Vyvanse Warning Letter: Too Late! Shire Got Rid of Ty Pennington Long Ago!“).
But, more importantly, the FDA clearly reviewed the storyboards for this ad long BEFORE it was aired. In fact, FDA offers the storyboards as evidence along with the warning letter on its website. I’ve captured part of the storyboard that shows the balloons:
 My question is this: If the FDA is going to preview broadcast DTC ads AND get paid to do it, why didn’t the agency stop the YAZ ad from being produced or aired after seeing this storyboard? Is FDA THAT slow in issuing warning letters? Or is it in cahoots with drug companies to help them avoid complying with regulations? Who needs a regulatory agency that regulates AFTER the fact and not before?
My question is this: If the FDA is going to preview broadcast DTC ads AND get paid to do it, why didn’t the agency stop the YAZ ad from being produced or aired after seeing this storyboard? Is FDA THAT slow in issuing warning letters? Or is it in cahoots with drug companies to help them avoid complying with regulations? Who needs a regulatory agency that regulates AFTER the fact and not before?
This can only get WORSE when drug companies routinely pay for FDA to preview their ads.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




