The pharmaceutical industry’s efforts to self-regulate its direct-to-consumer (DTC) advertising are “an industry-sponsored ruse,” intended to deflect criticism and collectively block new Federal regulation, a study released today in the Journal of Health Politics, Policy and Law found. The paper, “The Politics and Strategy of Industry Self-Regulation: The Pharmaceutical Industry’s Principles for Ethical Direct-to-Consumer Advertising as a Deceptive Blocking Strategy,” which you can find here, was written by Denis Arnold, Associate Professor of Management and Surtman Distinguished Scholar in Business Ethics in the Belk College of Business at UNC Charlotte, with Jim Oakley, Associate Professor of Marketing at Montana State University.
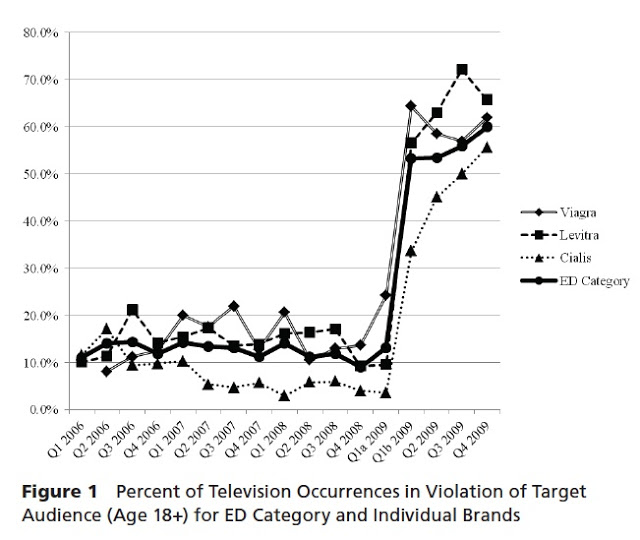
Arnold and Oakley studied the marketing campaigns for erectile dysfunction (ED) drugs — Viagra (Pfizer), Cialis (Eli Lilly), and Levitra (Bayer Healthcare, GlaxoSmithKline and Merck) — over a four-year period, 2006 to 2010. They found that a substantial percentage of TV ads for these products violated Principle 13 (audience composition) in the original 2006 “PhRMA Guiding Principles”, which requires that audience composition be 80 percent adult (eighteen years and older) for advertisements with adult-oriented content. This principle was in effect from January 1, 2006, to March 1, 2009. Here’s a chart from the study:
According to PhRMA’s revised guidelines, it is now expected that 90 percent of the audience for these advertisements will be eighteen years or older. “At no point have the three brands in this category successfully complied with this guideline at a rate better than 50 percent for the total category,” contend the authors of the study.
According to some experts, self-regulatory guidelines such as PhRMA’s DTC guidelines represent a “bridging” strategy in which firms take the proactive internal measures necessary to meet external expectations. “This might be accomplished by meeting and exceeding regulatory requirements or by voluntarily implementing self-regulatory standards within an industry,” note the authors of the study.
Self-Regulation as a Collective Blocking Strategy
The authors of this study, however, characterize what ED drug advertisers are doing as a “blocking” strategy, which “occurs when a firm publicly characterizes itself as engaged in bridging activity but is in reality engaged in activity that blocks unwanted external constraints on its activities. When a firm engages in a blocking strategy, it represents itself as responding proactively to social expectations about firm behavior in a manner consistent with a bridging strategy
but does not implement the internal measures necessary to ensure that the bridging strategy is implemented.”
The authors go even further, accusing the industry of engaging in a deceptive collective “blocking” strategy, which has the following components:
- it is facilitated by an industry trade group via an industry-wide
code of conduct, - it is a response to the perceived threat of additional regulatory oversight,
- the code of conduct is routinely violated by companies that have agreed to follow the code, and
- the code of conduct is not enforced by the trade group.
comments about company compliance with the principles.
“We submitted comments by mail on March 22, 2010. After six weeks and no response, we resubmitted our comments by mail and obtained confirmation of delivery on May 6, 2010, by the
US Postal Service. We also made repeated efforts to submit comment forms by facsimile beginning in May 2010, but the incoming calls at the number provided by PhRMA were not picked up by a facsimile machine
on different days and at different times. We continued this effort until
July 23, 2010, when on the eleventh attempt a facsimile was confirmed as
delivered. In the meantime, comments were again mailed to the PhRMA
Office of Accountability on July 13, 2010. No response to any of these
comments was ever received from PhRMA or from Bayer Healthcare, Eli
Lilly, GlaxoSmithKline, Schering Plough, Merck, or Pfizer as of December
27, 2012.”
They should have known this would happen after my own attempts to submit comments to PhRMA resulted in a strange delayed response from the famed “PhRMA Intern” (see “PhRMA’s Response – PRwise, it Stinks!“). Recall the envelope that the response came in:
PhRMA’s “Office of Accountability” is the ultimate “cockblocker” for complaints about violations of it DTC Guiding Principles.
The Adventures of PhRMA Intern!









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




