Perhaps 27% of the 229 dermatology-related mobile applications (apps) that are available today may be subject to FDA regulation despite the agency’s “enforcement discretion” safe harbor, which exempts most mobile apps that “pose minimal risk to consumers.”
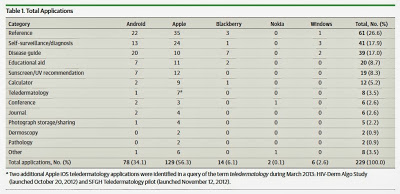
A study by Ann Chang Brewer, M.D., of the University of Arizona, Phoenix, and colleagues, published today in JAMA Dermatology ((JAMA Dermatol. Published online September 25, 2013. doi:10.1001/jamadermatol.2013.5517), identified 229 dermatology-related apps in several categories, including: general dermatology reference (61), self-surveillance/diagnosis aids (41), disease guides (39), educational aids (20) and sunscreen/UV (ultraviolet light) recommendations (19). Of the 229 apps, more than half were free.
The following table from that study gives the percentages:
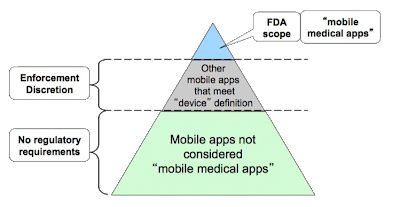
According to FDA’s Final Guidance on Mobile Medical Applications (here), “FDA intends to exercise enforcement discretion” for mobile apps that:
- Help patients (i.e., users) self-manage their disease or conditions without providing specific treatment or treatment suggestions;
- Provide patients with simple tools to organize and track their health information;
- Provide easy access to information related to patients’ health conditions or treatments;
- Help patients document, show, or communicate potential medical conditions to health care providers;
- Automate simple tasks for health care providers; or
- Enable patients or providers to interact with Personal Health Record (PHR) or Electronic Health Record (EHR) systems.
I believe at least three categories of dermatology apps fall outside FDA’s “enforcement discretion” safe harbor:
- Self-surveillance/diagnosis (18%)
- Calculator (5%)
- Teledermatology (4%)
“Self-surveillance/diagnosis apps varied in capabilities,” note the authors, “with some allowing patients to document lesions, upload and receive dermatologist or algorithm-based feedback about the malignancy potential of lesions, follow diagnosis algorithms, log personal treatment regimens, and/or record symptoms to allergen exposures.”
“Applications included in the calculator category provided quick calculations for medication dosing, laser fluence levels, or dermatology- related indexes, such as the Psoriasis Area Severity Index” (e.g., see “FDA Issues Long-Awaited Guidance – for Mobile Medical Apps. Janssen, Look Out!”).
The authors warn patients and clinicians to maintain a healthy sense of skepticism about the apps. They note studies regarding the safety and accuracy of such apps are limited and misdiagnoses from the apps could harm patients by potentially delaying treatment for conditions such as melanoma.
Considering the potential for harm, IMHO, the dermatology apps highlighted here should be elevated to the apex of the FDA’s mobile app regulatory scope triangle depicted above.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




