I heard that a number of participants at the 33rd Annual J.P. Morgan Healthcare Conference in San Francisco were “baffled” by LAP-BAND’s social media campaign. LAP-BAND, marketed by Apollo Endosurgery, is a medical device that is inserted surgically around the stomach to reduce its capacity and thus aid in weight loss.
LAP-BAND has a limited indication: “for weight reduction for patients with obesity, with a Body Mass Index (BMI) of at least 40 kg/m2 or a BMI of at least 30 kg/m2 with one or more obesity-related comorbid conditions.” It also has a number of possible adverse events such as “band slippage, erosion and deflation, reflux, obstruction of the stomach, dilation of the esophagus, infection, or nausea and vomiting may occur.”
It seems that several tweets posted by the @LAPBAND Twitter account violate recent FDA “Guidance for Industry
Internet/Social Media Platforms with Character Space Limitations— Presenting Risk and Benefit Information for Prescription Drugs and Medical Devices” (here).
First, here’s what the @LAPBAND Twitter page looks like:
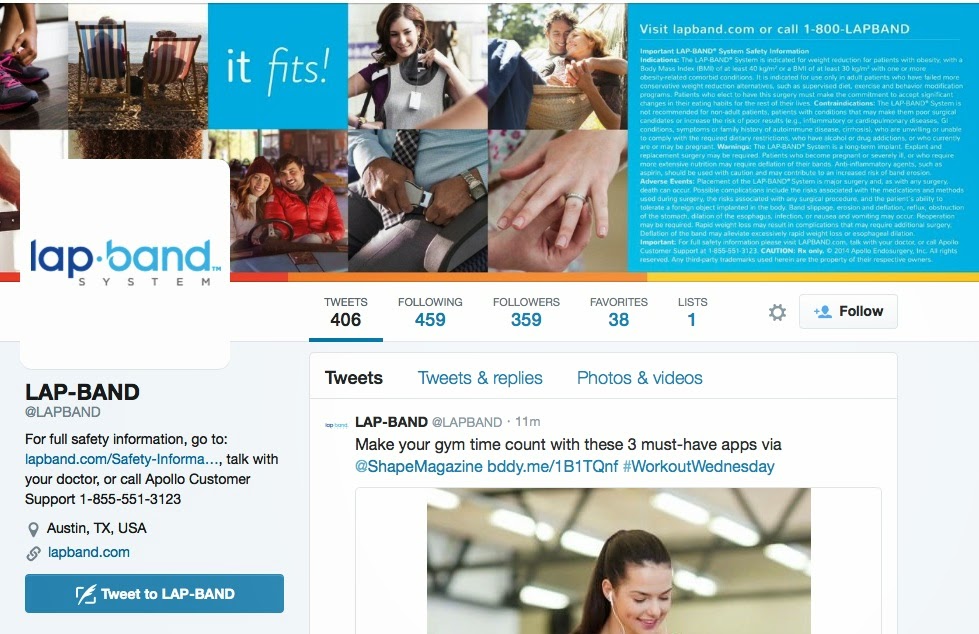 |
| Click on image for an enlarged vie. |
You can SEE – but you probably can’t READ – the Important Safety Information (ISI) in the upper right corner. Technically, I suppose this satisfies FDA requirements that ISI must accompany branded Rx and medical device ads that mention benefits of the product.
But it’s the tweets themselves that FDA should be looking out. Let me explain why.
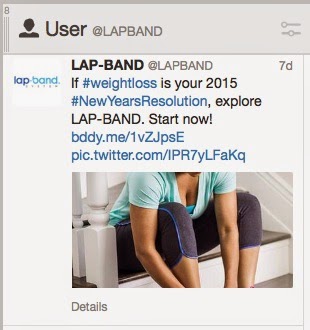
If you scroll down the @LAPBAND Twitter page, you find tweets such as the following:
These tweets mention the product brand name (“#LAPBAND”) and the indication (“lost 260 lbs.” and “#weightloss”). Should these tweets that also include the minimal ISI as per FDA’s guidance document mentioned above? The ISI is at the top of the page where these tweets are displayed, but you have to scroll up to see it.
NOTE: Since the Twitter handle is the name of the product (LAP-BAND), the brand name is actually part of every tweet. Thus even tweets that don’t include LAPBAND or LAP-BAND in the body of the tweet, must include safety information if it mentions weight loss.
Is there a “One Scroll Away Rule?”
So, technically you have the ISI “one scroll away” and that’s probably OK with the FDA — there’s precedent: many Rx drug websites have ISI at the bottom of the page and you have to scroll down to see it. So far, FDA has not sent any letters about that, so the “One Scroll Away Rule” is received precedent.
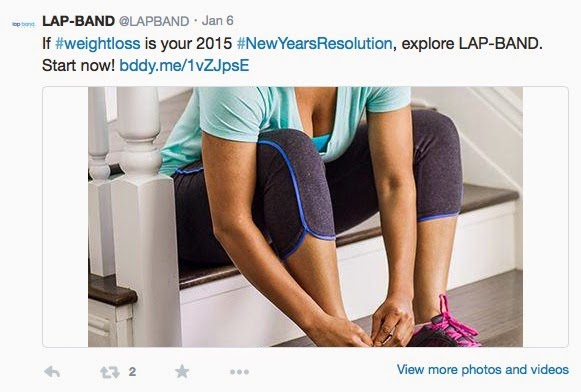
BUT… Twitter is different because most people probably do not view these tweets directly on the @LAPBAND Twitter page. Like me, many consumers view tweets on their mobile phones using the Twitter app, which does not display the whole page, or via Tweetdeck. Here’s how the tweet above looks on Tweetdeck (also on iPhone):
No ISI. Not any safety information at all! Clearly FDA should be looking at the @LAPBAND social media campaign and writing Apollo Endosurgery a Warning Letter.
BTW, you really have to scroll down a LOT on the www.lapband.com site to see the ISI. Just sayin’









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)