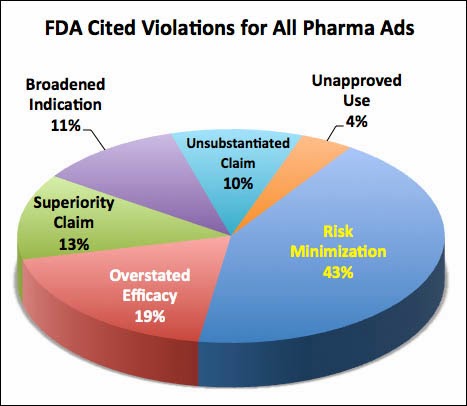
Balancing drug benefits (efficacy) against risks in direct-to-consumer (DTC) advertising has always been a contentious issue between pharma marketers and the FDA. About 40% of violations in drug ads cited by the FDA between 2004 and 2013 relate to “risk minimization.” That’s twice the percentage of violations relating to “overstated efficacy” (see chart).
From that you might conclude two things: (1) FDA is overly concerned with risk information in DTC ads, and/or (2) marketers have a difficult time packing in all the FDA-required risk information in these ads.
When space or time is limited — as in TV DTC ads — the drug industry is concerned that the major statement (see definition) of risks — as currently implemented in DTC ads — is often too long and “may result in reduced consumer comprehension, minimization of important risk information and, potentially, therapeutic noncompliance due to fear of side effects.”
When the industry is concerned, the FDA acts. However, before the FDA issues guidelines that may alter how it views risk information in TV ads, the agency will do a study (see “FDA Looking at Changing TV Advertising for Drugs as You Know It“).
In the Federal Register announcement of the proposed study (here), FDA reveals some ways that it may allow drug companies to substantially shorten the litany of risks in TV drug ads. Would these changes be in the best interest of patients or of drug marketers?
The FDA admits that there are viewpoints out there in conflict with the industry’s; namely, that “DTC TV ads do not include adequate risk information or leave out important information.”
As a possible resolution to these conflicting points of view, FDA posits (but does not yet propose) that TV DTC ads could “limit the risks in the major statement to those that are serious and actionable, and include a disclosure to alert consumers that there are other product risks not included in the ad.”
As an example, FDA cited this disclosure language: “This is not a full list of risks and side effects. Talk to your doctor and read the patient labeling for [drug name] before starting it.”
Read the “patient labeling”?! ROFL!
Back in 2006, the Coalition for Health Communication (CHC) petitioned the FDA to eliminate the need of “specific risk information in print and broadcast DTC” (see “DTC without the Risk“). CHC’s argument went something like this:
- Consumers are too dumb to weigh all the risks vs. benefits [CHC didn’t use the word “dumb.” Their petition used the more PC phrase “consumers with different educational and economic backgrounds”]
- Only prescribers — “learned intermediaries” — can do this
- DTC ads are effective at getting consumers to visit their doctors and ask about treatment and not effective as an educational tool
- DTC ads, therefore, should not mention specific risks, just say that there are risks and direct consumers to discuss these risks with their physicians.
The CHC even suggested wording for risk statements in DTC ads:
“Like all drugs, [drug name] has both benefits and risks. [Drug name] is only available by prescription, and your doctor can explain how [drug name] is likely to affect you. Be sure to tell your doctor about all of your medical conditions, and about any other medications you are taking, because this information could affect whether you should take [drug name]. Remember, only your doctor can decide if [drug name] is best for you.”
Although FDA does not seem ready to eliminate risk information entirely as recommended by the CHC, it does seem poised to take a step down a slippery slope.
Is this step even necessary?
First of all, pharma marketers have been citing evidence that TV DTC advertising is very effective with a 2:1 return on investment. So, these ads drive consumers to see their doctors, which is the goal of the ads.
FDA, however, implies that the risk information in the ads may cause patients to discontinue taking their drugs after seeing the ads and presumably after seeing their doctors and getting prescriptions.
How would reducing the risk info in ads change that?
Wouldn’t patients hear all about the risks from their docs (and from reading the “patient labeling”; ROFL!)?
Using FDA’s logic, to improve compliance it would be better to tone down the risk language in the labeling. That way neither physicians nor patients would be the wiser and patients would not get spooked and decline to take their meds. But that would be absurd.
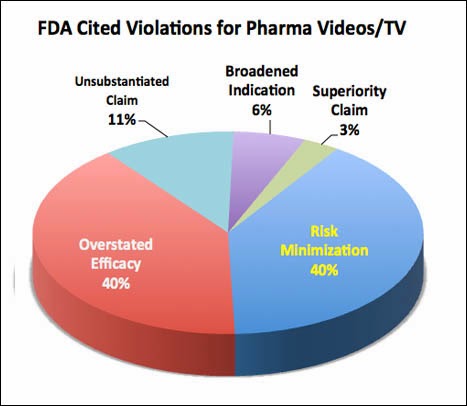
Also, from looking at FDA letters specifically citing violations in video and TV ads, there are an equal number of “overstated efficacy” and “risk minimization” citations in these letters (see chart above), which suggests that TV ads with less risk information would tip the balance too far toward benefits; i.e., the ads would emphasize the benefits over the risks to an even greater degree than they do today.
Listen to this audio snippet and podcast to learn more about overstatement of efficacy in TV DTC ads — a bigger problem, IMHO, than overstating the risks.
| How to Recognize Misleading Claims Made by DTC TV Ads | |
 In this 3.5-minute audio snippet, Adrienne E. Faerber, PhD, and David H. Kreling, PhD, talk about the key results of their content analysis of claims made by TV direct-to-consumer (DTC) drug ads and advise consumers how to recognize potentially misleading or outright false claims — including celebrity endorsements — made by these ads. In this 3.5-minute audio snippet, Adrienne E. Faerber, PhD, and David H. Kreling, PhD, talk about the key results of their content analysis of claims made by TV direct-to-consumer (DTC) drug ads and advise consumers how to recognize potentially misleading or outright false claims — including celebrity endorsements — made by these ads.
Your browser does not support the audio element. Upgrade your browser to one that does You can hear the full interview of Faerber and Kreling here: |
|








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




