A new study by researchers from Cambridge Health Alliance/Harvard Medical School, Boston Medical Center (BMC)/Boston University School of Medicine (BUSM), City University of New York School of Public Health, and Public Citizen, reveals that drugs released after the 1992 enactment of the Prescription Drug User Fee Act (PDUFA), which allowed the FDA to collect fees to expedite drug approvals, were more likely to be withdrawn or have a black box warning, with 26.7 percent of these drugs receiving such a warning compared to 21.2 percent in the pre-PDUFA drugs that underwent the longer approval process (see press release here).
“The FDA is under constant pressure to rush new drugs through the pipeline to approval. In its hurry, the FDA is apparently failing to distinguish useful drugs from toxic ones, and more dangerous drugs are slipping through,” said study lead author Cassie Frank, MD, a physician at Cambridge Health Alliance and an instructor in medicine at Harvard Medical School. “By the time many drugs receive serious safety warnings, millions of Americans have already been exposed to their side effects, which can sometimes be fatal.”
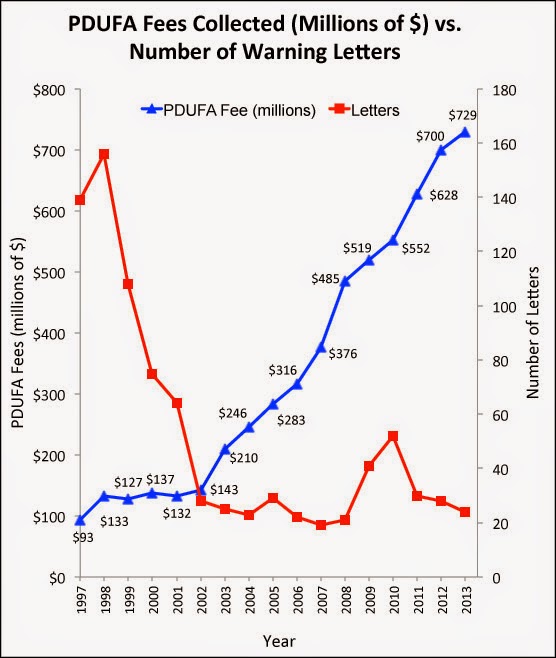
In recent years, the number of warning letters issued by the FDA regarding Rx drugs has dramatically decreased. Some experts claim that this is due to fewer drugs being approved and marketed. However, is it possible that the rise in PDUFA payments — which now account for about 65% of FDA’s budget for regulation of drugs — discourages the FDA from monitoring drug promotion and issuing warning letters?
Here’s a chart showing the increase in PDUFA funding and decrease in the number of warning letters from 1997 through 2013:
After 2002, when PDUFA fees really started to shoot up, the number of warning letters issued per year was significantly less than in previous years.
The Government Accounting Office (GAO) submitted testimony that documented, among other things, how long it took the FDA to issue regulatory letters citing violative DTC materials during Troy’s reign. As the chart below shows, there has been a definite trend that correlates with the appointment of Troy.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




