The deadline has come and gone for submitting comments to docket FDA-2009-N-0441 regarding “Promotion of FDA-Regulated Medical Products Using the Internet and Social Media Tools.” You can find ALL of the comments here at Regulations.gov and easily download MOST of them here at fdaSM.com. Unfortunately, the latter does NOT include text comments that were submitted by individuals. You can find these at the end of this post.
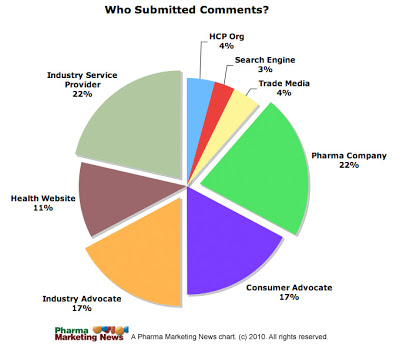
Seventy (70) different entities submitted one or more comments to the docket. These submitters can be organized into the following eight general categories:
- HCP Org – Health Professional (HP) or HP Assn
- Search Engine – Google and Yahoo!
- Trade Media – Blog, newsletter, publication focused on drug industry news
- Pharma Company – Drug, device, or diagnostic company
- Consumer Advocate – Individual or consumer advocacy group
- Industry Advocate – Trade association or ad hoc group
- Health Website – Patient, physician, or health activist focus
- Industry Service Provider – Marketing, communications, ad or PR agency that provides services to drug industry
Here’s the pie chart representing the distribution (click on it for enlarged view):
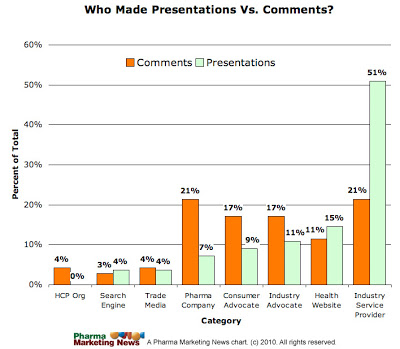
I also compared the types of entity that submitted comments to those that made presentations at the November 2009 public hearing. Of course, there was some overlap because many did both. Here is the bar chart showing the comparison:
(See “Industry Groups will Eat Consumer Advocates’ Lunch at FDA Social Media Public Hearing” for my analysis of entities that made presentations at the public hearing.)
It’s immediately obvious that there is a more even distribution among commentators than among presenters. Whereas only 7% of presenters at the public hearing were pharma companies, 21% of commentators were pharma companies. Pharma obviously played it close to the vest and many companies decided not to be in the limelight at the public hearing but waited until the very last minute to submit comments to the docket.
Consumer Advocates also stepped up to provide comments, whereas very few made presentations at the public hearing. As I suggested at the time, it cost money to attend these hearings and individuals simply cannot afford it.
My “Consumer Advocate” category of commentators is comprised of these 12 organizations and individuals:
- Anonymous, Individual
- Bruce Overman Jr, Individual
- Center for Digital Democracy, Consumer Advocacy Group
- Consumers Union, Consumer Advocacy Group
- Kathryn Rowerdink, Individual
- Kathy Lambert, Individual
- Melvin Flowers, Individual
- Michael E. Bailey, Individual
- National Organization for Rare Disorders, Consumer Advocacy Group
- Randall Pecsek, Individual
- Michael E. Bailey, Individual
- PEW Prescription Project, Consumer Advocacy Group
Ten (83%) of these expressed ANTI-pharma marketing sentiments such as the following:
“I think the implications of this can get out of hand. Social media and the internet allows for too much false, partially true, information which can harm the lay people who do not know the difference. It may lead to unapproved/remakes of devices that are not safe.” — Anonymous
“I and many, many others are very much against any further promotion or advertising of Food and Drug Administration-Regulated Medical Products, particularly prescription drugs. I am very much in favor of outlawing the existing practice of advertising prescription drugs. Billions of dollars are spent by pharmaceutical companies to advertise drugs, confusing and misleading the public, most of whom do not have the expertise needed to make proper judgments where these drugs are concerned. This massive amount of money should, instead, be used to reduce the cost of these drugs.” — Bruce Overman Jr
“By using an array of new digital marketing tools — including behavioral targeting, social media, online video, and mobile — pharmaceutical companies now have unprecedented abilities to take advantage of consumers.” — Center for Digital Democracy
“The experience with DTC ads from 1997 to the present should be a cautionary tale and compel the agency to carefully examine its options and the appropriate mechanisms to assure against widespread and unbalanced promotion of drug products via online media.” – Consumers Union
“I do not want Rx drugs advertised or promoted on TV or through media to the public.” — Kathy Lambert
“This is surely helpful use the FDA regulatory authority. There need to be control on the Internet advertisements epically when it relates to medical devices and labeling.” — Melvin Flowers
“The manufacturers of medications and their representatives must be held accountable for each claim they put on any online media concerning their products because the public health and safety demands it. There maybe some online media that are not suited for drug advertising because of the space limitations involved. There may not be enough space for the important risk information that needs to come with the claims. It is too risky and dangerous to allow the drug company claims alone without the risk information. It is not enough to put in a link that you can click on to take you to another site to get the risk information because many people won’t do that and will only read the claims of the drug company that it puts up on the social media. But they should always provide a link to the FDA website for people who want an unbiassed and fair assessment of the drug, and so people can report bad reactions to the drug. Thank you and best wishes, Michael E. Bailey.”
“The last thing this country needs is MORE advertising by drug companies. Prescription drugs need to be administered by doctors acting in the best interest of their patients. Patients need to talk to their doctor about a medical “problem”, and let the doctor determine the best treatment. Drug ads serve only to feed hypochondria in the public. Marketing of drugs and the costs of advertising serve only to drive up prescription drug costs in America. Our capitalist system is out of control, driving Americans to spent money frivolously. Let’s not expand advertising opportunities, let rein them in and recind the rules which allow drug companies to advertise anywhere except medical journals intended for doctors. How many more erectile dysfunction ads do we need to be subjected to?” — Randall Pecsek
“[W]e urge caution in promulgating rules that could effectively establish new and pervasive modes of industry marketing. It is important that current FDA guidance on the presentation of risk information not be compromised to the detriment of the public health in favor of accommodating evolving industry marketing practice. If a marketing tool, such as a space-limited microblog, or tweet, is unable to satisfy basic consumer protective measures such as the fair balance requirement, that tool should be considered inappropriate for the promotion of pharmaceutical products.” — PEW Prescription Project
“To reduce demand and help curb online diversion, we urge FDA to more stringently regulate pharmaceutical companies’ advertising of controlled prescription drugs to physicians and consumers.” — The National Center on Addiction and Substance Abuse, Columbia University
The two “PRO” commentators expressed these opinions:
“Patients and patient organizations increasingly are using the social media as an important communications tool. We expect this trend to continue. Patients and families affected by rare diseases have a great need for information since they typically have a greater-than-usual participation in their own, or their loved one?s, disease management and treatment. There is opportunity for the social media to perform a helpful role in facilitating the exchange of information for rare-disease patients and families. There also is significant potential for misuse of the social media or for the circulation of inaccurate or misleading information. It is NORD?s recommendation at this time that FDA continue its deliberation of how best to provide guidance regarding manufacturers? participation in the social media, since the use of social media for sharing of medical information is widespread and growing. We understand that FDA?s resources are limited. However, it is our sense at this time that clear guidance from FDA, similar to that which is currently being provided for traditional advertising modalities, would help to define appropriate ways for manufacturers to participate in this newest of the new media. If NORD, as the primary representative of the rare disease patient community, can assist FDA with defining what the guidelines should be, we would be happy to work with FDA on that process.” — National Organization for Rare Disorders
“I believe transparency is requested for all of the healthcare industry. Prior to twitter, facebook etc. the public was posting comments on drugs (pros and cons). I have googled drugs by their marketed and generic names to read about other peoples’ experiences. I had some reactions to a steroid medication I was prescribed and wanted to find others who might have experienced the same. Doing a search and finding the right forum was extremely difficult and time consuming. A drug makers facebook page or a separate page for each drug was available, it would have made life much easier. A Facebook etc with non-censored updates, stories and comments etc. would have helped me and been faster. If the drug companies and insurance companies (although this is not the topic for the FDA)agree not to censor comments and stories, this is e a great avenue for information exchange. The pros and cons of a drug are more easily assessed through other peoples experiences and comments. Regulations and laws have forced the drug industry to use very complex wording in the packaging (in good faith of full disclosure) however this not helpful to the consumer but overwhelming. I would much rather go to a social media site and view what the possible tangible experiences are so that I may weigh my options and assess the risk. If the FDA chooses to regulate social media I do not see how this is a move toward transparency. The more the FDA represses communication avenues the less people feel informed. Regulating the censoring of comments etc. should be enforced but there are too many loopholes in trying to prevent the healthcare industry from engaging in new communication avenues. The FDA should encourage communication between patients and drug makers. Not only will this help the drug companies to assess needs and fill gaps but consumers are more likely to make their voices heard and feel empowered.” — Kathryn Rowerdink
With individuals such as “Kathryn Rowerdink,” it’s not clear if they have a bias based on whether or not they receive funding indirectly from the drug industry (eg, are employed by an ad agency that has drug company clients).








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




