
I once believed that pharmaceutical research (part of R&D) was the “bright” side of the pharmaceutical industry and that marketing was the “dark” side (see “God Bless R&D, but Marketers May Go to Hell!“).
Then, as so often happens, some pharmaceutical company does something stupid like rigging clinical trial end points to benefit marketing (see “Pharma R&D Succumbs to the Dark Side“).
Pharmaceutical companies may be rigging clinical trials in another way: by carefully recruiting patients who are most likely to benefit from the treatment. New evidence in support of this “dark” side of R&D is presented in the latest issue of JAMA.
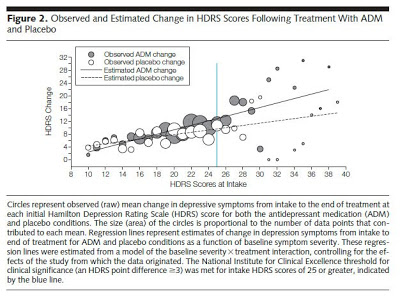
In the article, “Antidepressant Drug Effects and Depression Severity: Patient-Level Meta-analysis”; JAMA. 2010;303(1):47-53 (doi:10.1001/jama.2009.1943), the authors find that antidepressants are no better than placebo for most depressed patients (ie, those with mild, moderate, or “even severe” baseline symptoms; see chart below; click on it for an enlarged view).
“What makes our findings surprising,” say the authors, “is the high level of depression symptom severity that appears to be required for clinically meaningful drug/placebo differences to emerge, particularly given the evidence that the majority of patients receiving ADM [antidepressant medication] in clinical practice present with scores below these levels.”
What about all those clinical trials trials that “prove” effectiveness? It turns out that to be eligible for these trials, patients must have pretty high depression scores (HDRS greater than 18). These are precisely the patients most likely to benefit from treatment. “Such cutoffs,” note the authors, “can be expected to exclude nearly half of all patients who meet diagnostic criteria for MDD [major depressive disorder].”
But it is not likely that marketers of antidepressants point this out to physicians, especially not to general practitioners who often prescribe antidepressants.
As the authors note: “This important feature of the evidence base [lack of effectiveness in all but the most severe cases] is not reflected in the implicit messages present in the marketing of these medications to clinicians and the public. There is little mention of the fact that efficacy data often come from studies that exclude precisely those MDD patients who derive little specific pharmacological benefit from taking medications.”
I’m wondering about other drugs — Viagra, for example. How were those clinical trials designed? See “Pfizer’s Erection Hardness Meter” for more about that!








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




