In preparation for FDA’s public hearing on the Promotion of Food and Drug Administration-Regulated Medical Products Using the Internet and Social Media Tools, the agency is asking for comments on 19 specific questions (see “Let’s Respond to FDA’s Questions Regarding Its Regulation of Social Media“). These questions are included in my ongong online survey/questionnaire, which you can access here.
I am following the results of this survey closely and will provide updates. Here, I focus on this question:
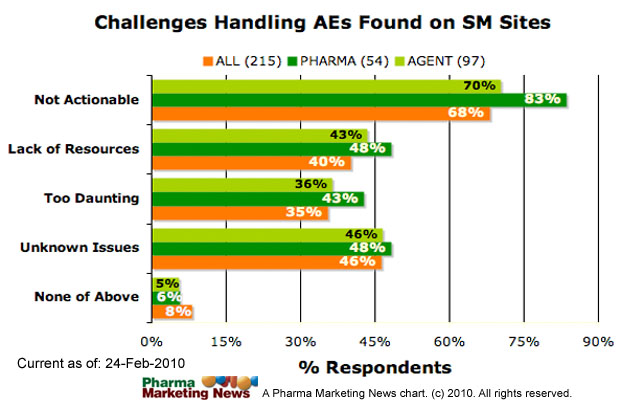
What challenges are presented in handling adverse event information from these sources?
The survey asks respondents to choose one or more of the following responses (and/or add additional comments):
- The amount of information from these sources is potentially too vast to be processed economically (lack of resources)
- Finding adverse event information from these sources is like finding a needle in a haystack (too daunting)
- The information is usually incomplete and does not meet the requirements for submitting a meaningful AER (not actionable)
- There are many potential issues that won’t fully be known until the practice of monitoring social media for AEs is more prevalent (unknown issues).
- None of the above
The image below shows how respondents answered this question.

SPECIAL REPORT: FDA Regulation of Social Media
Find links to more results here.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)