In a couple of days I will be leaving for the “Pharmaguy European Tour 2013.”
I will visit Berlin, Munich, and Milan and return on June 21, 2013. Part of my time will be spent meeting “old” friends like LenStarnes (@lenstarnes) and making new friends like Dr. Jesus del Valle (@yeysus), project leader of the Bayer HealthCare Grants4Apps™ (@grants4apps) program.
Bayer HealthCare Grants4Apps™ invites health app developers to submit their innovative app ideas for novel software that contributes to improving health outcomes or pharmaceutical processes. Bayer HealthCare considers an “App” as software solution based on any platform. Selected projects will be supported with an amount of either 5.000€ or 10.000€.
Dr. del Valle invited me to speak at a “Bayer Meets Startups!” event, Tuesday, 11 June, in Berlin. The title of the event is “Bayer Pharma Meets Pharmaguy Meets [Healthcare App] Startups.” I will give an updated presentation that focuses on Overcoming Pharma’s Social Media & Mobile Challenges.
It’s a great opportunity for me to meet innovative health app developers in Germany and see if they are anything like the “typical” mobile app developer I described previously on Pharma Marketing Blog (see “Is This the Typical Mobile Health App Developer Hired by Pharma?“). I am guessing that many health app innovators in Germany and elsewhere do not know much about pharma industry restrictions and regulations that may apply to their apps (see, for example, “More Mobile Health App Guidance from FDA for Pharma to Worry About“).
More importantly, I can promote my ideas for pharma self-regulatory Mobile Health App “Guidelines.” In addition to fretting about what the final FDA mobile medical app guidelines will look like, the pharmaceutical industry needs to be pro-active in assuring the public that the mobile health apps it develops are of the highest quality. Already, there are problems with pharma apps that had to be recalled (see “The First Ever “Dear Doctor” Letter Regarding a Mobile Medical App Recall“).
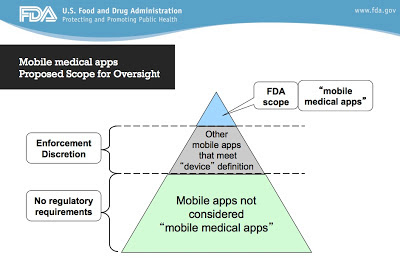
As illustrated in the following FDA “Scope of Oversight” Pyramid, there are health apps that are NOT MMAs (“mobile medical apps”) and therefore not in FDA’s regulatory scope. But even “out-of-scope” apps should be of the highest quality and comply with best practice standards established by the industry.
Just as the pharma industry has developed guidelines for direct-to-consumer advertising and interactions with healthcare professionals, it needs to develop guidelines for consumer and HCP-focused health apps. These guidelines should address issues like privacy, transparency, accuracy, certification, and documentation.
If you are interested in giving me your opinion about this, I invite you to take my “Pharma Mobile Health App Best Practices Survey” (here).
I will try and tweet during my tour, which also includes a visit to Roche Italy’s Digital Academy in Milan (see here). I will definitely share what I have learned when I return.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




