It’s unusual for a pharmaceutical company to mention a product by brand name on its corporate blog. It’s even more unusual to mention BOTH the product AND its indication — because that would be promotion regulated by the FDA. But AstraZeneca (AZ) has done just that on its “AZ Health Connections” corporate blog.
The majority of the post “New CDC data shows drop in number of adults with high cholesterol” submitted by Tom Hushen, AZ’s External Communications Manager, talks about CRESTOR, AZ’s anti-cholesterol drug. The post may have been ghostwritten for “Dr Philip de Vane, Executive Director of Clinical Development at AstraZeneca,” whose name appears at the bottom.
After briefly citing the results of the CDC (Centers for Disease Control) study (see below) in the first paragraph, Hushen dedicates the most of the remaining 309 words of the 377-word post to CRESTOR as in:
“AstraZeneca applauds this progress and we are proud that when diet and exercise alone aren’t enough, prescription medications like CRESTOR® (rosuvastatin calcium) are able to help patients reach their cholesterol goals. In adults, CRESTOR is prescribed along with diet to lower high cholesterol and to slow the buildup of plaque in arteries.”
Included in the post is the “fair balance” information required by law:
“CRESTOR is not right for everyone─like people with liver disease or women who are nursing, pregnant or may become pregnant. Tell your doctor about other medicines you are taking. Call your doctor right away if you have muscle pain or weakness; feel unusually tired; have loss of appetite, upper belly pain, dark urine, or yellowing of skin or eyes─these could be signs of rare but serious side effects. See www.CRESTOR.com”
Although this is not earth-shaking or in violation of any law that I know of, it nevertheless is the FIRST time a pharmaceutical company has promoted a prescription drug on its official corporate blog — ie, talked about the drug’s benefits.
It’s even more interesting considering the AZ Health Connections “Comment Policy” seems to preclude any comments about specific products:
“We want to make sure AZ Health Connections provides a good experience for all visitors. Therefore, we want to keep the content focused on the specific topics being addressed. Comments that don’t directly relate to AstraZeneca or the topics currently being discussed, or comments or questions about specific products (whether or not AstraZeneca products) or ongoing legal or regulatory matters may not be published or may be removed.”
Could it be that what’s good for the “goose” (AZ) is not good for the “gander” (everyone else)? It seems that AZ has relaxed its comments policy, at least this one time. As proof of this, I submitted the following comment, which AZ published:
“I am one of those U.S. adults with high cholesterol that is having problems controlling it with just diet and exercise, which I don’t even try to do :-). But I am worried about taking powerful medicines such as CRESTOR because of the side effects that you mention.”
AZ published that comment made by this “gander.” It is the only comment published so far, so I have no idea if other people have submitted comments that were NOT published. Maybe Tony Jewell, Senior Director of External Communications at AstraZeneca US, will tell us. NOTE: Jewell received the coveted “Pharmaguy Social Media Pioneer Award” in 2011 (see here).
Note: In a personal email, Jewell said: “This post was reviewed, as are all others that mention medicines or disease states. There have been many on the blog, Twitter and Facebook.” Upon searching the AZ blog site for other posts that mention CRESTOR, I could not find any post that mentioned the product name AND the disease state (high cholesterol) it is approved to treat. There were, however, a couple of posts that mentioned CRESTOR without its indication.
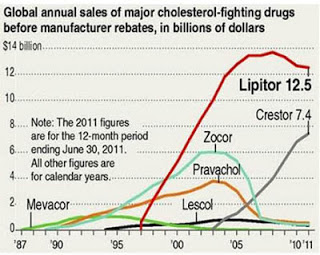
Why did AZ do this at this time? It seems to be very opportunistic considering that it coincides with the release of CDC data that shows improvement to cholesterol levels for many Americans. Also, Pfizer just announced it is no longer promoting Lipitor (see “Pfizer Throws In the Lipitor Marketing Towel” and “Lipitor R.I.P. Infographic“).
Obviously, now is a good time for AZ to ramp up the promotion of CRESTOR, as it is positioned to take over the number one (or virtually ONLY) statin TOP sales spot (see chart below):
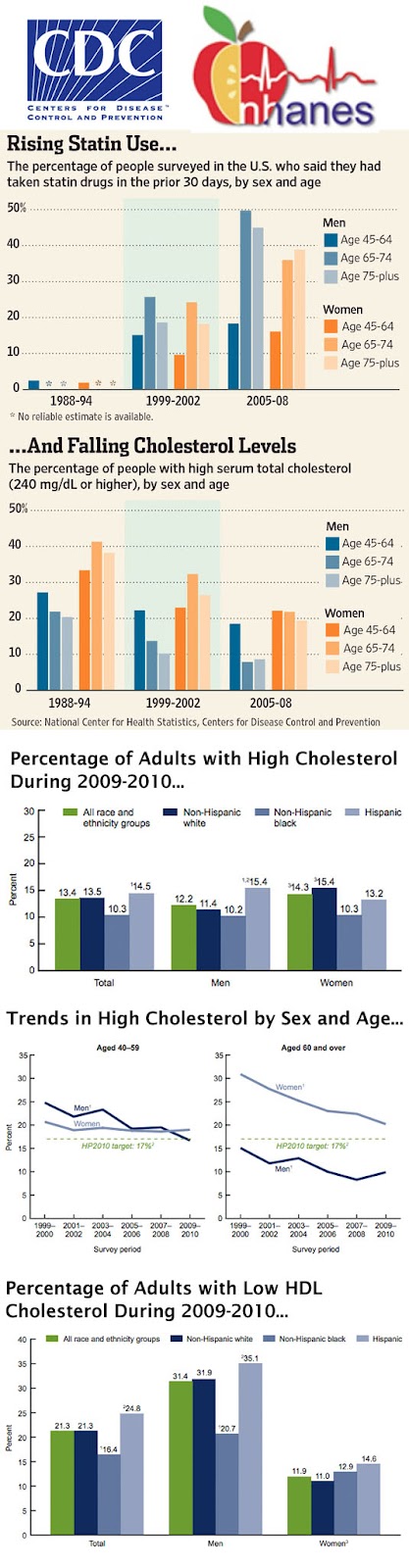
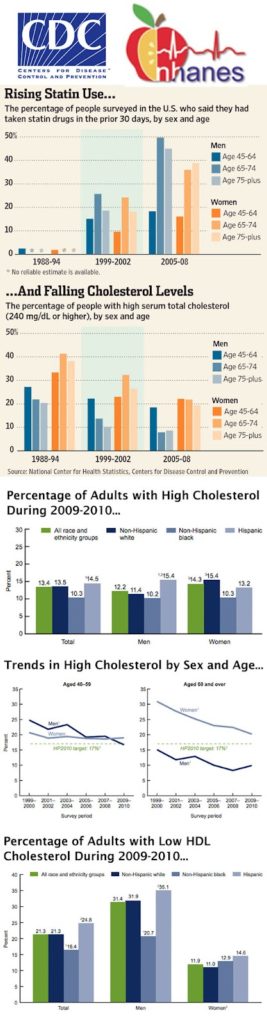
It’s also obvious that AZ wants to take some credit for the results reported by the CDC, which are summarized in the following “infographic” (creating infographics is a new obsession of mine):
Some interesting conclusions can be made from the data in this CDC report, which you can find here.
For one thing, women are not doing as well as men in terms of lowering their total cholesterol. This is especially true for women aged 60 and over. Women in that age group have consistently higher percentages of high total cholesterol than men.
The percentage of adults with low HDL cholesterol was higher for men (31.4%) than for women (11.9%).
Although the CDC does study the use of statin drugs by adults and breaks this down by sex and age (see top chart in the infographic), the CDC’s analysis that is highlighted in AZ’s blog post is based “only on measured cholesterol and does not take into account whether medications are taken.”
That’s too bad. It would have been interesting to see the correlation between statin use and lowered measured cholesterol.
More important than managing cholesterol levels, however, is whether or not statins actually improve health outcomes such as heart disease. There are results from clinical trials that indicate such a benefit, but how does that correlate with results in the real world?
Just curious.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




