In April, the FDA sent warning letters to several pharmaceutical companies citing paid search engine ads that it claimed violated FDA regulations (see “FDA’s Actions Speak Louder than Its Words: On the Internet It’s the Medium as Well as the Message!“).
But what about organic — ie, unpaid, “non-sponsored,” natural — search engine listings such as the following for Viagra (click on image for an enlarged view)? Are they next on FDA’s chopping block?
That was a question posed by Julie Batten, e-marketing manager at Klick Communications (see “Search Marketing in the Pharma Industry“).
“To date, organic listings haven’t been considered promotional, and rightly so,” said Batten.
Batten notes that pharma companies can determine the wording of organic listings by carefully crafting “meta” data (ie, TITLE and DESCRIPTION) within the header of HTML files that generate Web pages. Since “meta data isn’t meant to be consumer-facing; it’s meant to be [Search Engine] spider-facing,” Batten argues that organic listing based on meta data “aren’t within the jurisdiction of the regulating bodies to review and approve.”
However, upon review of a few meta tags for Rx drug sites, I find that the wording seems to be crafted especially for consumers and not just for search engines. The following are some examples:
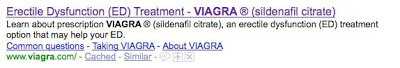
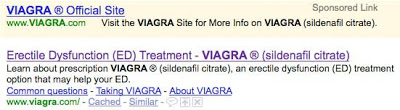
Viagra
- Meta tag TITLE: “Erectile Dysfunction (ED) Treatment – VIAGRA ® (sildenafil citrate)”
- Meta tag DESCR: “Learn about prescription VIAGRA ® (sildenafil citrate), an erectile dysfunction (ED) treatment option that may help your ED.”
Sponsored ad (top) vs. organic listing (bottom):
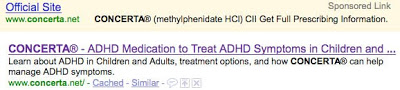
Concerta (http://www.concerta.net)
- Meta tag TITLE: “CONCERTA® – ADHD Medication to Treat ADHD Symptoms in Children and Adults”
- Meta tag DESCR: “Learn about ADHD in Children and Adults, treatment options, and how CONCERTA� can help manage ADHD symptoms.”
Sponsored ad (top) vs. organic listing (bottom):
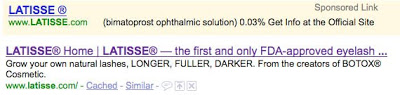
Latisse
- Meta tag TITLE: “LATISSE® Home | LATISSE® — the first and only FDA-approved eyelash growth treatment”
- Meta tag DESCR: “Grow your own natural lashes, LONGER, FULLER, DARKER. From the creators of BOTOX® Cosmetic.”
Sponsored ad (top) vs. organic listing (bottom)
In each case, the organic listing exactly matches the meta tags, which are clearly written with the searching consumer in mind. That is, each DESCR meta tag is actually a small direct-to-consumer (DTC) ad! However, none of these descriptions contains the fair balance (ie, side effect) information that FDA requires real-world DTC ads to include.
So, my question is: Will the FDA look upon organic search engine listings as direct-to-consumer ads because the meta tags are clearly written as ads, not merely descriptions of the landing page?









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




