
Allergan, the specialty pharma company BEST known for its marketing of BOTOX, was voted the “Most Admired Specialty Company” in the Eighth Annual Med Ad News Most Admired Companies contest/poll/popularity contest.
Allergan achieved this distinction among Med Ad News readers who were polled by the publication despite the fact that the company does not abide by PhRMA’s DTC Guidelines that prohibit “reminder ads.” Reminder ads mention the pharmaceutical brand name but not the indication or medical condition it treats (see definition here).
ALL BOTOX direct-to-consumer print and TV ads are reminder ads, which are free to imply outlandish benefits — such as “freedom of expression” (see “Botox BS Piled Higher and Deeper“) — without any counterbalancing risk information as required by FDA regulations simply because FDA is not empowered or inclined to regulate reminder ads.
Most of the rest of the drug industry have ceased to run reminder ads.
I also notice that Allergan is not a signatory to PhRMA’s new Guidelines for Interactions with Healthcare Professionals (see PMN Reprint #77-01). Allergan sales reps, therefore, are likely flooding dermatologists’ offices with logoed pens, pads, and other “tchotckes.”
All this while Solstice Neurosciences, the company that markets Myobloc, a BOTOX competitor, is playing by PhRMA rules.
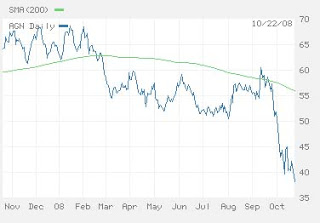
But I’m not only complaining that Allergan is admired for its audacity to side-step industry best practices. What startled me when reading the glowing 2-and-one-half page spread dedicated to Allergan in the October 2008 issue of Med Ad News was that BOTOX was mentioned only briefly, in a short paragraph, and not in bold text as were other products that were discussed at length. There was no mention of any problems with FDA (see below) and no sales trend data for Botox. Every other product Allergan EVER made and marketed or has in the pipeline WAS mentioned in great detail! Keep in mind that BOTOX accounts for about one-third of Allergan’s 2007 $3.9 billion in sales and Allergan is currently seeking approval for a new BOTOX indication (migraine).
 “Joe the Investor” is Misled
“Joe the Investor” is Misled
BOTOX sales performance has recently taken a “hit” due to FDA investigations and competition.
In February 2008, the FDA issued an “Early Communication” about BOTOX safety issues and began investigating reported deaths attributed to BOTOX use (see “Early Communication about an Ongoing Safety Review Botox and Botox Cosmetic (Botulinum toxin Type A) and Myobloc (Botulinum toxin Type B)“). That hurt sales and Allergan’s stock performance.
Obviously, this story did NOT get included in Med Ad News’ glowing account of Allergan. Instead, the story focused on Allergan’s 2007 sales and stock performance. I suspect, in fact, that the Med Ad News article was written by Allergan itself — there’s no byline indicating the author as in other articles in the publication.
Considering the dismal performance of Allergan’s stock over the past year, the Med Ad News article does a disservice to “Joe the investor” by presenting a biased view of a company with low marketing standards. If it was written by Allergan, shouldn’t the SEC required disclaimer be included?
Tomorrow, I hope, Chris Truelove will provide the Med Ad News side of the story in her Pharma Blog Review blog.
UPDATE: Christine Truelove responds…
“John Mack at the Pharma Marketing Blog had some complaints about Med Ad News‘ writeup of Allergan as Most Admired Specialty Company. Well, Mr. Mack, here’s the explanation. Med Ad News didn’t pick Allergan as Most Admired Specialty Company — our readers did, in online polling. But Allergan didn’t supply the writeup; Managing Editor Andrew Humphreys did, from examining Allergan’s own press releases and reports. Since the readers picked Allergan as the most admired among the top 25 specialty drug companies by revenue, he had to write about why they might have done so. Yes, we know about the falling sales of Botox and FDA investigations; I believe our readers do as well. Yet, they still picked Allergan, which meant we had to focus on the things that may have spurred their decision. As Mr. Humphreys put it to me, “My job is not to point out the problems for the companies that are voted as winners, because all companies have issues in one way or another. This story was about what makes them admirable. We have other forums to discuss issues such as drug advertising regulation.” By the way, I doubt “Joe the Investor” is reading us; our circulation is to the pharma and marketing industries and some academics. But your criticisms are being taken constructively; they’ve given me some ideas as to change the format of these writeups for next year.
Stay tuned …”
Thanks for your response, Chris. I’m happy that you are taking this as constructive criticism regarding future writeups of your survey winners — and, yes, I know Med Ad News readers voted on this and not the editors, a fact that I specifically mentioned in the second paragraph of my post.
I am surprised, however, that you don’t consider “Joe the Investor” as a reader. Many people working in the pharma and marketing industries — your core readers as you say — also invest in the drug industry. Drug company “Joe” and “Jane” employees get stock options or buy their company’s stock as part of their 401K plans. Many people may also diversify their holdings by buying stock in drug companies other than their own. Then there are all the Med Ad News readers that work for companies that service the pharmaceutical industry. I am sure many of them are also interested in investing in the industry.
Maybe Allergan’s employees willingly drink the Allergan corporate Koolaid that appears in the press releases that your editor copies, but other pharma and service industry employee/investor readers of your magazine deserve a more balanced view.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




