 I read with interest Jim Edwards’ recent story about how “Allergan’s Eyelash Enhancer Makes Your Blue Eyes Brown.”
I read with interest Jim Edwards’ recent story about how “Allergan’s Eyelash Enhancer Makes Your Blue Eyes Brown.”
It appears that Allergan’s strategy for expanding the market for Lumigan — its anti-glacoma drug — is to capitalize on one of the drug’s side effects: patients’ eyelashes become longer when using it!
Of course, many women love long eyelashes and spend a lot of money on cosmetics and even permanent tattooing to make their lashes longer or seem longer. In fact, according to Edwards, doctors are already using Lumigan eye drops off-label for vanity purposes (more on that below).
But there’s another side effect of Lumigan that may make it useful to terrorists and other criminals who seek to fool security systems that depend on iris recognition technology — Lumigan can change the color of your eyes (eg, make blue eyes brown). Of course, this side effect would only be useful for blue-eyed terrorists and, as we all know, most terrorists throughout history — excepting Nazis, of course — have been dark-eyed.
Jim noted this problem and mentioned that one of his “cosmetic dermatologist sources said upon learning of the effect, ‘I must tell my mafia clients!'”
Mafia. How quaint! Does the FBI still go after the “mafia”? And, let’s not forget that most mafia types are of the dark-eyed genotype.
In any case, if you are like thousands of Med Ad News readers who think that Allergan is a pharma company to be admired (see “Allergan Doesn’t Comply with PhRMA Guidelines, Wins Kudos Anyway“), then you probably admire Allergan for its creativity in attempting to turn a sow’s ear of a drug into a silk purse! [“Lipstick on a pig” may be the more appropriate, but less politically-correct, metaphor!]
Not that there’s anything wrong with that — many drug companies have benefited from exploiting side effects of drugs meant for other purposes (eg, Pfizer and Viagra) — but Allergan often does not play by the rules as I pointed out in the blog post I cited in the previous paragraph.
I suspect, however, that Allergan’s strategy is NOT to seek FDA approval of Lumigan for eyelash enhancement. For one thing, it is too ridiculus to waste FDA’s precious time reviewing such a trivial indication for a drug. But the main reason I say this is because Allergan has a history of relying on off-label use and avoiding marketing rules.
For example, Edwards reminds us that Allergan is under investigation by the Department of Justice for alleged off-label promotion of Botox for headache (see “The Allergan-Medicis Vanity Pharma Death Match Is About to Get Complicated“).
And, for what it’s worth — and according to several reader comments it’s not worth much — neither Allergan nor Medicis is a signatory of PhRMA’s new guidelines for interactions with healthcare professionals. Both companies are free to wine and dine dermatologists and convince them to use their products off-label.
The clincher, however, comes from Allergan itself. If you look for any clinical trials for the use of Lumigan for eyelash enhancement on the Allergan site, you’ll find bupkis, nada, zilch. On Allergan’s Medical Aesthetics ongoing clinical trial web page you’ll find “No studies currently recruiting at this time.”
No trials are necessary either for certain dermatologists who are pretty upfront about using Lumigan off-label. Here’s the pitch one dermatologist makes on her Web site: “Achieve Luxurious Lashes with Lumigan®. Only available through physicians this miracle solution stimulates your own eyelashes to grow longer and thicker. Easy to use at home with only a once daily application.” The following photos are offered as evidence of Lumigan’s effectiveness in enhancing lashes:
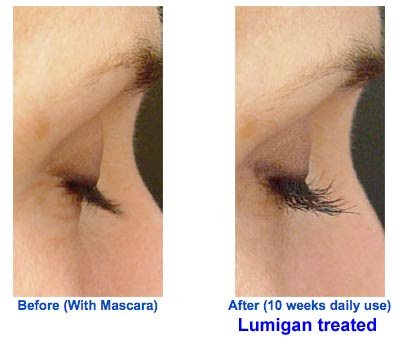 P.S. The dermatologist mentioned above is Lorrie Klein, M.D., who claims to be one of the “Top 10 Botox & Juvederm Injectors in United States!”
P.S. The dermatologist mentioned above is Lorrie Klein, M.D., who claims to be one of the “Top 10 Botox & Juvederm Injectors in United States!”
OMG! She must get lots of perks from Allergan AND Medicis! No doubt she’s shooting to achieve Top 10 status among “Lumigan Droppers” too!








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




