New data from the FDA (here) shows that 2014 was a banner year for orphan drugs, which are drugs that treat “rare” diseases/disorders affecting fewer than 200,000 people in the U.S.
The drug approval data from 1994 through 2014 (to date) are plotted in the following charts:
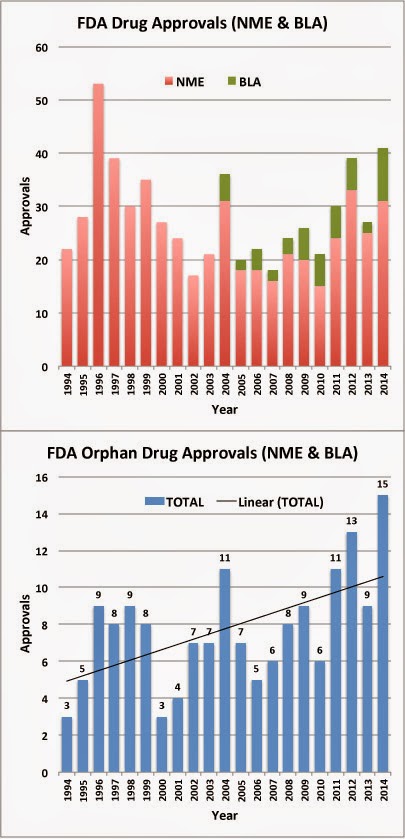 |
| NME=New Molecular Entity, BLA=Biologic License Application; Source: FDA |
Let’s dig deeper into the numbers.
A total of 41 new molecular entities (NMEs) and biologics (BLAs) were approved by the FDA in 2014 – 15 of those were approved in December alone (11 in final two weeks!). That’s the highest number of approvals since 1996.
Orphan drug approvals represent 37% (15 out 41) of all new drug approvals in 2014. Today, orphan drugs have the potential to turn into blockbusters with annual sales over $1 Billion (read, for example, “New Big Pharma Economies of Scale: Less Patients Needed to Reach Blockbuster Sales“).
2014 was also a very good year for biologics. Ten (10) new licenses to market biologic drugs were issued to date in 2014. That’s more than any other year and represents 24% of all new drug approvals.
[This post was updated on 2-JAN-2015 with new data.]








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




