In preparation for FDA’s public hearing on the Promotion of Food and Drug Administration-Regulated Medical Products Using the Internet and Social Media Tools, the agency is asking for comments on 19 specific questions (see “Let’s Respond to FDA’s Questions Regarding Its Regulation of Social Media“). These questions are included in my ongong online survey/questionnaire, which you can access here.
I am following the results of this survey closely and will provide updates. Here, I focus on this question:
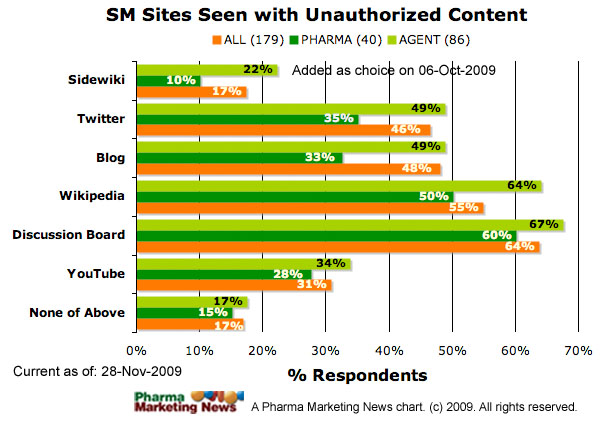
With regard to the potential for company communications to be altered by third parties, what is the experience to date with respect to the unauthorized dissemination of modified product information (originally created by a company) by noncompany users of the Internet?
The survey asks respondents to choose one or more of the following responses (and/or add additional comments):
- Unauthorized product information pages in Wikipedia
- Unauthorized product Twitter accounts
- Unauthorized product Blogs
- Unauthorized communications in discussion boards
- Unauthorized Google Sidewiki comments on drug.com sites
- None of the above
The image below shows how respondents answered this question (ALL respondents vs. PHARMA respondents vs. Agency respondents). The image will be updated frequently. See the date stamp on the image for when it was last updated. To see the most up-to-date results, please take the survey yourself and you will be able to see a summary at the end.

SPECIAL REPORT: FDA Regulation of Social Media
Also see:
- What Criteria Determine Substantive Pharma Influence Over Content on Social Media Sites? Survey says…
- Should FDA Regulation Depend on Specific Media or Audiences? Survey says…
- 3rd Party Dissemination of Altered Rx Drug Information on Social Media Sites. Survey says…
- Pharma Companies Should Have Public Social Media Disclosure Policies, Survey Results Show
- Pharma Companies Should Have Public Social Media Disclosure Policies, Survey Results Show









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




