In 2014, the FDA approved 41 new drugs — the most since 1996 (see here). That’s quite a “record” even if 37% of the approvals were made in December alone.
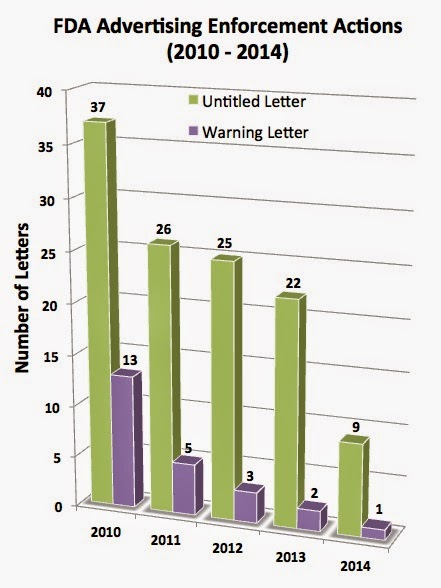
But FDA wasn’t as anxious to set any advertising enforcement records in 2014. The Agency sent out only a meager 9 Untitled Letters and 1 Warning Letter last year (see chart on left).
Could the reason for this be that pharmaceutical marketers are getting better at complying with FDA regulations?
Or is the FDA afraid of being sued by the pharmaceutical industry in the wake of the 2012 Caronia decision in the U.S. Second Circuit that found off-label promotion was protected by free speech?
Or perhaps FDA depends more and more on “user fees” paid by the drug industry and does not want to “bite the hand that feeds it”?
I think the latter is the most likely explanation. Read on to learn why.
It’s difficult to believe pharma marketers are getting better at complying with regulations when in recent years one drug company after another has agreed to pay multi-million/billion dollar settlements to the DOJ for inappropriately, and in some cases illegally, promoting prescription drugs (see here, for example). Also, the industry began learning to comply with FDA regulations long ago starting in 1997-1998 when direct-to-consumer (DTC) advertising began in earnest. So this does not explain the recent drop in letters
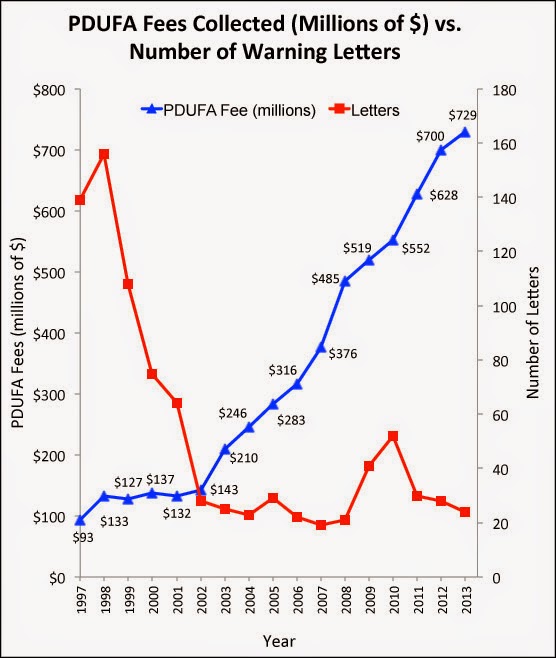
With regard to off-label claims, none of the letters issued in 2014 mentioned that violation. Historically (between 2004 and 2013), only 4% of violations cited in FDA letters was concerned with off-label (“unapproved use”) promotion (see here). So, the 2012 Caronia decision probably is not a factor in why fewer and fewer letters have been issued by FDA every year beginning in 1998. Note: There was a “spike” in 2009 and 2010, which was probably in reaction to the VIOXX fiasco (see chart below).
The chart above shows that since 2002 PDUFA fees collected by the FDA have increased dramatically, which corresponds with the historically flat number of untitled/warning letters issued in the following years (except for the VIOXX spike mentioned above).
I contend that in the years prior to the rise in PDUFA fees, FDA issued fewer and fewer warning letters so as not to upset the political process authoring the future collection of the fees. Since then it has been more or less laissez-faire and the money continues to roll in.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




