Vol. 10, Issue No. 8: 28 APRIL 2011 – EXECUTIVE SUMMARY Physicians Favor Brands With a Compelling Adherence Program Highlights from a Physician Survey by HealthPrize Technologies
 An estimated one third to one half of all patients in the U.S. do not take their medication as pre-scribed by their doctors. Any shift in moving the needle towards better adherence is a win-win scenario that benefits both patients and drug brands.
An estimated one third to one half of all patients in the U.S. do not take their medication as pre-scribed by their doctors. Any shift in moving the needle towards better adherence is a win-win scenario that benefits both patients and drug brands.
HealthPrize uses a patented “Engagement Engine” to reward patients for taking their medication. In order to gauge the value of its adherence solution to physicians, HealthPrize sponsored an independent survey of physicians designed to assess the value of various adherence solutions — including HealthPrize — and their potential to influence physicians’ prescribing behavior.
This article summarizes the survey results, which were first presented during a Pharma Marketing Talk podcast interview of HealthPrize’s Chief Medical Officer, Katrina S. Firlik, M.D., and John Ruvane, HealthPriz’s VP of Sales and Business Development>
Topics include:
- Physician Buy-in is Key to Success
- Drug Parity Exits, Say Physicians (includes chart)
- Chart: Importance of Prescribing Influencers
- Key Findings of the Study
- HealthPrize Dashboard
- Chart: The Potential Impact of HealthPrize on Physicians’ Preference
- Adherence as a Core Product Benefit
- Transcript of Interview
Read this article now. It’s FREE…
Implications of Facebook’s Page Commenting Changes The “Final” Story
 A discussion with Jonathan Richman, Group Director, Insights and Planning at Possible Worldwide about Facebook’s changes to its commenting policies and his recommendations for pharma marketers who wish to develop Facebook pages.
A discussion with Jonathan Richman, Group Director, Insights and Planning at Possible Worldwide about Facebook’s changes to its commenting policies and his recommendations for pharma marketers who wish to develop Facebook pages.
Read the article and listen to the podcast here:
http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/news/pmn108-article02.htm
A New Estimate of Drug Development Cost Economic Experts Disagree Wildly on How Much It Costs to Bring a New Drug to Market.
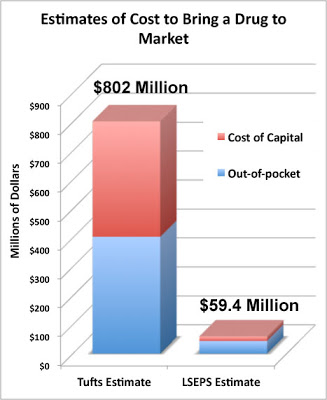 How is it that the Tufts estimate is at least 14 TIMES the London School of Economics and Political Science (LSEPS) estimate?
How is it that the Tufts estimate is at least 14 TIMES the London School of Economics and Political Science (LSEPS) estimate?
“Make no mistake,” said Tufts. “Tufts CSDD will vigorously defend the scholarship, integrity, and validity of all its published research studies.”
Read more about this here:
http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/news/pmn108-article03.htm FDA to Test New Standard for Easy to Understand Drug Labels Patients also need more effective counseling about their medications.
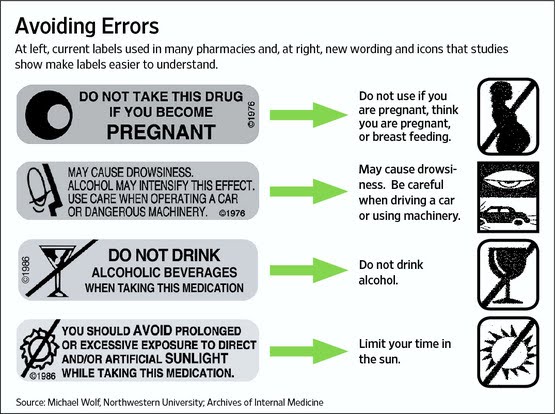 In an effort to make drug package inserts easier to read and understand — and perhaps to save money on printing costs — the FDA is planning to test single-page consumer information sheets that would replace the multi-page package inserts and medication guides widely used in retail pharmacies. However ‘dumbed down’ the label gets, it is still important that physicians and pharmacists take a more pro-active role in educating consumers about the drugs they are taking.
In an effort to make drug package inserts easier to read and understand — and perhaps to save money on printing costs — the FDA is planning to test single-page consumer information sheets that would replace the multi-page package inserts and medication guides widely used in retail pharmacies. However ‘dumbed down’ the label gets, it is still important that physicians and pharmacists take a more pro-active role in educating consumers about the drugs they are taking.
Find more results of the survey here:
http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/news/pmn108-article04.htm Digitally-Feeble Pharma Is Your Brand a Digital Genius or a Feeble-Minded Idiot?
 When it comes to digital IQ, some brands are geniuses and some are feeble-minded idiots, according to the “L2 Digital IQ Index” for pharmaceutical brands, a first-of-its kind measurement of the digital competence of 51 pharma brands across eight therapeutic categories.
When it comes to digital IQ, some brands are geniuses and some are feeble-minded idiots, according to the “L2 Digital IQ Index” for pharmaceutical brands, a first-of-its kind measurement of the digital competence of 51 pharma brands across eight therapeutic categories.
Read more about this here:
http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/news/pmn108-article05.htm



![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-218x150.jpg)




![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




