Vol. 10, Issue No. 6: 30 MARCH 2011 – EXECUTIVE SUMMARY

SURVEY: Use of Twitter by Pharma Employees
CONTENTS
(Click on titles to see a summary and link to each item)
- BREAKING NEWS: It’s Official! FDA Will Miss Its Q1 2011 Goal for Issuing Social Media Guidance.
- Up Front: Adverse Event Reporting: A Missed Opportunity
- Pharma TeleWeb e-Detailing
- Pharma Twitter Pioneers Recognized
- Physicians Favor Brands with Compelling Adherence Platform
- Are Sales Reps as “Useful” as PhRMA Wants Us to Believe?
- Astellas Scientist Shows How to Light Ice on Fire!
- FDA Promises Still More Guidance! This Time It’s Mobile.
Article Summaries Up Front
Adverse Event Reporting: A Missed Opportunity
 When asked what the # 1 concern pharmaceutical marketers have regarding using social media, the adverse event reporting (AER) boogey man is cited most often.
When asked what the # 1 concern pharmaceutical marketers have regarding using social media, the adverse event reporting (AER) boogey man is cited most often.
Many marketers hope the FDA will issue guidance to help them neutralize the boogey man, but that is not likely to happen any time soon. In fact, I’ve heard that DDMAC won’t be issuing those guidelines (see, for example, http://bit.ly/gMDxmC), but has handed the job over to another division of the agency. In my mind that does not bode well — at least DDMAC is the devil we know!
Meanwhile, a recent Supreme Court ruling may have greater repercussions for adverse event reporting by the drug industry than any guidelines the FDA may issue.
Read this entire OpEd piece by John Mack here:
http://www.news.pharma-mkting.com/PMNews_106_Upfront.pdf Pharma TeleWeb e-Detailing
A New Media Role for Sales Reps
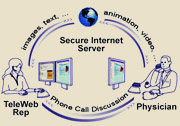 Physician detailing remains a dominant channel for drug promotion. It accounts for 55% of the total pharma promotional spend (69% if you take samples out of the equation). Although the total spend on detailing is decreasing, there is a continued shift from traditional detailing to e-detailing.
Physician detailing remains a dominant channel for drug promotion. It accounts for 55% of the total pharma promotional spend (69% if you take samples out of the equation). Although the total spend on detailing is decreasing, there is a continued shift from traditional detailing to e-detailing.
“Traditional forms of promotion, such as face-to-face detailing and meetings, are becoming less commonplace, while internet media such as e-detailing and e-meetings are growing at a fast and steady pace,” according to Cegedim. “Tele-detailing” accounts for 49% of the new media channel physician promotional spend and “TeleWeb e-Detailing,” which combines phone and Web site, is a growing trend.
This article summarizes Eli Lilly’s experience using TeleWeb e-detailing in Europe.
Topics include:
- Promotional Mix Depends on the Brand
- New Media Promotion Increasing
- Tele-Detailing is Prevalent
- Chart: Promotional Spending Trends by Channel
- Chart: Top Brands in Promotion Spending
- Table: Top Companies in Growing Channels (2010)
- Chart: New Media Average Monthly Spending 2006-2010
- A Lilly Case Study
- Key Performance Indicators
- TeleWeb and Face-to-Face Call Synergies
- Will TeleWeb Calls Replace Field Calls?
Order and pay for this reprint now using your credit card…
ONLY $4.95Download PDF file immediately after paying:
Pharma Twitter Pioneers Recognized
Employees with Personal Twitter Accounts
 More and more pharmaceutical company employees are using Twitter. Many are using personal Twitter accounts and some also are responsible for corporate Twitter accounts. These employees can be influential ambassadors among the public and have an opportunity — maybe even a responsibility — to help improve the company’s reputation.
More and more pharmaceutical company employees are using Twitter. Many are using personal Twitter accounts and some also are responsible for corporate Twitter accounts. These employees can be influential ambassadors among the public and have an opportunity — maybe even a responsibility — to help improve the company’s reputation.
Last year, @pharmaguy recognized pharma social media pioneers based on their efforts to implement social media marketing campaigns or to champion social media awareness and knowledge within their companies. This year, Pharmaguy is looking at pharma employees who have personal Twitter accounts, how they use these accounts, who follows them and whom they follow, and how influential they are.
This article is an introduction to the first round of members of this group.
Topics include:
- Can Twitter Improve Pharma’s Reputation?
- Social Media Guidelines for Employees
- Who Is Tony Jewell?
- Table: List of Pharmaguy Twitter Pioneers
- How to Qualify to Be on the List
- Pioneer “Klout”
- Chart Showing the Number of Twitter Followers of Pharmaguy Twitter Pioneers
- Who Do Pioneers Follow?
- Chart: Pharmaguy Twitter Pioneer Followers vs. Following
- PeerIndex
- More Twitter Pioneers Sought
Download this article (pdf) here:
http://www.virsci.com/pmn/PMN105-01twpi.pdf
Physicians Favor Brands with Compelling Adherence Platform A conversation with Katrina S. Firlik, M.D., Chief Medical Officer, and John Ruvane, Vice President, Sales and Business Development, HealthPrize, highlighting results of an independent physician survey that suggests HealthPrize’s patient adherence program can sway physician prescribing preferences.
A conversation with Katrina S. Firlik, M.D., Chief Medical Officer, and John Ruvane, Vice President, Sales and Business Development, HealthPrize, highlighting results of an independent physician survey that suggests HealthPrize’s patient adherence program can sway physician prescribing preferences.
The 100-physician survey, designed to assess the value of adherence solutions and their potential to influence physicians’ prescribing, found that the addition of a rewards-based medication adherence solution shifted reported prescribing preferences between two competing drugs by over 30 percentage points.
Some Questions/Topics Discussed
- How can a brand use an adherence program as a marketing tool?
- Do you anticipate that these results will translate into a real-world market share shift?
- Is HealthPrize better suited for a product in a particular stage of its lifecycle? Launch vs. facing patent expiry?
- Do you see HealthPrize complementing or competing with other brand initiatives designed to promote loyalty — i.e., co-pay cards, CRM initiatives, etc.?
Listen here: http://bit.ly/PMT132
Are Sales Reps as “Useful” as PhRMA Wants Us to Believe?![]() “New Survey Emphasizes Value of Biopharmaceutical Company Engagement With Healthcare Providers” is the main point PhRMA (Pharmaceutical Research and Manufacturing Association – the industry trade association) emphasized in its press release regarding a survey of physicians it sponsored. PhRMA also pointed out that nearly 9 out of 10 physicians considered company-sponsored peer education programs to be “up-to-date, useful and reliable.”
“New Survey Emphasizes Value of Biopharmaceutical Company Engagement With Healthcare Providers” is the main point PhRMA (Pharmaceutical Research and Manufacturing Association – the industry trade association) emphasized in its press release regarding a survey of physicians it sponsored. PhRMA also pointed out that nearly 9 out of 10 physicians considered company-sponsored peer education programs to be “up-to-date, useful and reliable.”
That’s good news. But it’s always educational to go beyond the PR to learn something new, even some things the drug industry may not want you to learn.
What else does the survey reveal about the value of sales reps?
Read more here: http://bit.ly/PhRMAKRCsurveyAstellas Scientist Shows How to Light Ice on Fire! This is Robert Kernstock, Principal Scientist at Astellas Pharma US. I was alerted to meet Robert and “learn why he LOVES science & who inspired him” by @AstellasUS, the company’s Twitter account.
This is Robert Kernstock, Principal Scientist at Astellas Pharma US. I was alerted to meet Robert and “learn why he LOVES science & who inspired him” by @AstellasUS, the company’s Twitter account.
I immediately recognized Robert from a video he made that teaches kids how to light ice on fire. That video was the highlight of a conference I attended last year. That’s Robert and his fiery ice on the left.
The Astellas YouTube channel showcases several of its scientists as part of the company’s Science WoRx (get it? ie, “Rx” for prescription) project, which is a local mentoring program and online resource network for science teachers.
See the video here (Don’t try this at home!): http://bit.ly/IceOnFireFDA Promises Still More Guidance! This Time It’s Mobile.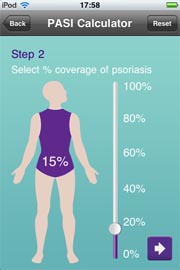 “The Food & Drug Administration is planning to release guidance for mobile medical applications in 2011, according to Center for Devices and Radiological Health director Dr. Jeffrey Shuren.”
“The Food & Drug Administration is planning to release guidance for mobile medical applications in 2011, according to Center for Devices and Radiological Health director Dr. Jeffrey Shuren.”
As you probably know, another divison of the FDA — DDMAC — has “promised” that it would issue social media guidance for pharmaceutical promotion by the end of Q1 20011. Considering the looming budget cuts suggested by some anti-government bloat lawmakers, it could be a cold day in hell before FDA can spare the resources to get the job done as “promised.”
Regarding mobile devices FDA has a much, much greater challenge in issuing guidance. The issue has to do with agency approval of Mobile medical applications as medical devices. So far, FDA has approved only two mobile applications
Meanwhile, many pharmaceutical marketers are developing mobile Apps such as Janssen’s “Psoriasis” app for the iPhone and iPad. Is this app a medical device that requires approval by the FDA?
Read more about this here: http://bit.ly/MedDevApp





![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-218x150.jpg)




![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




