FDA To Study DTC Promotion Directed at Adolescents Focus is on Immediate Benefits vs. Deferred Risks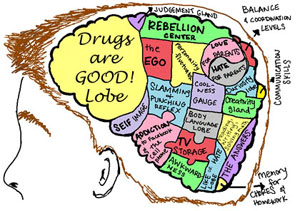 “Adolescents are a unique audience for DTC drug marketing because their cognitive abilities are different than those of adults,” noted the FDA in an October, 2013, Federal Register notice to conduct an “Experimental Study of Direct-to-Consumer Promotion Directed at Adolescents” (FR # FDA-2013-N-1151). Yet some pharmaceutical companies are promoting powerful drugs to teens on the Web and on TV.
“Adolescents are a unique audience for DTC drug marketing because their cognitive abilities are different than those of adults,” noted the FDA in an October, 2013, Federal Register notice to conduct an “Experimental Study of Direct-to-Consumer Promotion Directed at Adolescents” (FR # FDA-2013-N-1151). Yet some pharmaceutical companies are promoting powerful drugs to teens on the Web and on TV.
FDA’s proposed study will have adolescents and their parents view a DTC promotional campaign for either a fictitious Attention Deficit Hyperactivity Disorder (ADHD) medication or a fictitious acne medication. The study will explore similarities and differences in perceptions for these matched pairs. This article is a review of that study, which aims to assess how adolescents interpret drug risk in DTC advertising.
Topics include (partial list):
- Examples of Drugs Marketed to Teens
- FDA’s Research Into Teen-Targeted DTC Ads
- The Adolescent Brain
- Shire’s Comments
Download the full text PDF file here:
www.pharma-mkting.com/news/pmnews1310-article02.pdf
#FDA Needs to Do a Better Job Communicating DTC Research Results, Says Expert
PMN1310-02
Issue: Vol. 13, No. 10: December 2014



![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-218x150.jpg)




![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




