What’s at Stake in the Off-Label Debate Freedom of speech may be the legal issue, but enhancing the bottom line is the business issue In a study published in an April, 2011, issue of PLoS Medicine, Aaron S. Kesselheim from Brigham and Women’s Hospital, Boston, USA and colleagues allege that “despite Federal Drug Administration regulation of the approval and use of pharmaceutical products, ‘off-label’ marketing of drugs (for purposes other than those for which the drug was approved) has occurred in all aspects of the US health care system” (see “Off-label marketing of medicines in the US is rife but difficult to control“). The authors identified three main goals of alleged off-label marketing programs: expansion of drug use to unapproved diseases, expansion to unapproved disease subtypes, and expansion to unapproved drug dosing strategies, typically higher doses.
In a study published in an April, 2011, issue of PLoS Medicine, Aaron S. Kesselheim from Brigham and Women’s Hospital, Boston, USA and colleagues allege that “despite Federal Drug Administration regulation of the approval and use of pharmaceutical products, ‘off-label’ marketing of drugs (for purposes other than those for which the drug was approved) has occurred in all aspects of the US health care system” (see “Off-label marketing of medicines in the US is rife but difficult to control“). The authors identified three main goals of alleged off-label marketing programs: expansion of drug use to unapproved diseases, expansion to unapproved disease subtypes, and expansion to unapproved drug dosing strategies, typically higher doses.
According to a study published in Mayo Clinic Proceedings, many patients have no idea that they are taking a drug in a manner not reviewed by the FDA and doctors have no obligation to inform them, researchers stated (see “Ten Common Questions (and Their Answers) About Off-label Drug Use“).
The FDA defines off-label Rx drug use as, “use for indication, dosage form, dose regimen, population or other use parameter not mentioned in the approved labeling.” In the US, it is legal for doctors to write off-label prescriptions. Up until now, it has not been generally legal for pharmaceutical companies to promote any drugs they manufacture for off-label use.
Between January 1996 and October 2010, the U.S. government collected $7.9 billion from drug companies as part of settlements of off-label promotion cases brought against them. Recent court cases, however, are questioning the legality of FDA’s enforcement of its “blurry” off-label guidelines.
The Caronia Case
The most recent case was a 2-to-1 split decision by the 2nd U.S. Circuit Court of Appeals in New York that threw out the conviction of a sales representative for promoting off-label use of a prescription drug (see story here).
The majority decision written by Circuit Judge Denny Chin — the same judge that rejected Google’s deal to digitize books (see here) — was based on last year’s Supreme Court ruling that “Speech in aid of pharmaceutical marketing . . . is a form of expression protected by the Free Speech Clause of the First Amendment.” According to Judge Chin, the “pharmaceuticalrepresentative’s promotion of an FDA-approved drug’s off-label use is speech” and he was prosecuted “precisely” because of his “speech in aid of pharmaceutical marketing.” Ergo, “we VACATE the judgment of conviction and REMAND the case to the district court.”
There’s enough legal issues in this case to keep a ship-load of constitutional and pharma lawyers busy for years. But I’d like to look at this from a different perspective — i.e., what pharma has to gain from this and what patients have to lose.
This particular case involves several off-label discussions between a sales rep and a physician who recorded the conversations. The drug being promoted by the sales rep was Xyrem, a powerful central nervous system depressant, approved for “narcolepsy patients who experience cataplexy” and “narcolepsy patients with excessive daytimesleepiness.” Xyrem’s active ingredient is gamma-hydroxybutryate (“GHB”). GHB has been federally classified as the “date rape drug” for its use in the commission of sexual assaults. Xyrem can cause serious side effects and, if abused, it can cause additional medical problems, including seizures, dependence, severe withdrawal, coma, and death. It’s FDA labeling includes a “black-box” warning.
To promote this drug off-label, say for use in children under the age of 16, or for “restless leg syndrome” (which can be diagnosed without any evidence other than what the patient testifies to), might be considered by any sane person a danger to public health, akin to yelling FIRE! in a crowded theater when there is no fire.
But that is exactly what the sales rep did. On October 26, 2005, Caronia (the sales rep defendant in the case) “plainly promoted the use of Xyrem in unapproved indications with Charno (a physician who recorded the conversation as an agent for the FDA):
[Caronia]: And right now the indication is for narcolepsy with cataplexy . . .excessive daytime . . . and fragmented sleep, but because of the properties that. . . it has it’s going to insomnia, Fibromyalgia[,] periodic leg movement,restless leg, ahh also looking at ahh Parkinson’s and . . . other sleep disorders are underway such as MS.
[Charno]: Okay, so then so then it could be used for muscle disorders and chronicpain and . . .
[Caronia]: Right.
[Charno]: . . . and daytime fatigue and excessive sleepiness and stuff like that?
[Caronia]: Absolutely. Absolutely. Ahh with the Fibromyalgia.”
On separate occasions, Caronia and Gleason (the physician paid by the drug company) each explained to prospective physician-customers that Xyrem could be used with patients under age sixteen, an unapproved Xyrem subpopulation:
[Caronia]: Um, the youngest patients we have are sixteen in the studies as old assixty-five. Ahh there have been reports of patients as young as fourteen using itand obviously greater than sixty-five. It’s a very safe drug.
[Gleason]: Well, it’s actually approved for sixteen and above um, I’ve had peopleunder thirteen and I’ve certainly talked to neurologists that have narcoleptics. . . between eight and ten . . . [but] I start at two-thirds the dose, but [if]they’re real frail I only start with one-third the dose.
Pharma’s “Friend of the Court” Brief
Other than quoting the above conversation, Judge Denny Chin did not consider the safety of patients as part of his reasoning for reversing the lower court’s decision. And neither did the drug industry, which filed a “friends of the court” brief. “FDA’s regulations censor manufacturers,” says the Medical Information Working Group (MIWG), which filed the brief. MIWG is an “informal” group of pharmaceutical companies that includes Allergan, Amgen, Boehringer Ingelheim USA, Eli Lilly & Co., GlaxoSmithKline, Johnson & Johnson, Novartis Pharmaceuticals, Novo Nordisk, Pfizer, Purdue Pharma, and sanofi-aventis U.S.
Pharma Citizen Petition Calls for New Regulations
In July, 2011, Allergan, Eli Lilly, Johnson & Johnson, Novartis, Pfizer, Novo, and Sanofi-Aventis filed a “citizen petition” with the FDA, urging the agency to “establish comprehensive, clear and binding regulations to guide the industry” in communicating off-label drug information to physicians and payers.
The petition requests that FDA issue clarifying regulations on four separate, but related issues:
- Manufacturer responses to unsolicited requests — mostly requests for off-label information. Specifically asks for a “safe harbor” defining what constitutes a “non-promotional response.”
- “Scientific exchange” — specifically asks to clarify what FDA means by this
- Interactions with Formulary Committees, Payors, and Similar Entities — specifically asks for clarity on sharing health care economic and other drug information
- Dissemination of Third-Party Clinical Practice Guidelines — specifically guidelines that may discuss off-label uses of drugs.
Listen to this conversation with Darshan Kulkarni, Principal Attorney, Kulkarni LLC.
Listen to internet radio with Pharmaguy on Blog Talk RadioThe MIWG contends that a manufacturer that “speaks about the lawful off-label uses of its products subjects itself to potential enforcement action unless FDA and DOJ determine, in their sole discretion, that they will not treat the speech as evidence of an ‘intended use’ for the product. This creates a chill on manufacturers’ speech, which has serious potential consequences for physicians, patients, and the public health.”
Because of FDA’s regulations that “lack coherence and clarity,” MIWG claims physicians will have difficulty obtaining “objective, balanced, and accurate information on important unapproved uses of approved products.” Furthermore, MIWG contends that pharma companies are “uniquely positioned to provide physicians with such information.” (see “Drug Firms Push to End Ban on Off-Label Marketing“).
Judge Chin conceded that “While some off-label information could certainly be misleading or unhelpful, this case does not involve false or misleading promotion [my emphasis]. Moreover, in the fields of medicine and public health, ‘where information can save lives,’ it only furthers the public interest to ensure that decisions about the use of prescription drugs, including off-label usage, are intelligent and well-informed.”
That kind of thinking supposes that all information is helpful, whereas we know that a lot of information promulgated by many marketers, including pharmaceutical marketers, can be unsupported by evidence; i.e., some “information” can be “carefully wrought bullshit” (see “Is Pharma Marketing a Lot of BS?“).
Even the “evidence” that pharma companies use to “prove” the efficacy and safety of drugs is being questioned (see “The Case Against EBM“). But I rather not get into that here.
Dissenting Opinion
In her dissenting opinion, Circuit Judge Debra Ann Livingston was more focused on the safety issue: “one way in which a drug is misbranded is if its labeling lacks adequate directions for layperson use.” She points out that the FDA defines “adequate directions for use” as “directions under which the layman can use a drug safely and for the purposes for which it is intended.”
“But some uses of drugs are never safe, at least for a layperson,” said Judge Livingston, “because it is impossible to provide adequate directions for such uses, this provision also effectively prohibits introducing drugs into interstate commerce with the intent that the drugs be used in ways that are unsafe for laypersons… the simple fact that one is generally allowed to sell something does not imbue one with a constitutional right to sell it for any intended purpose. And the prohibition here on distributing drugs with the intent that they be used for purposes not supported by their labeling is entirely consistent with the broader purposes of the FDCA — namely, minimizing those occasions on which patients use drugs that have not been shown to be safe and effective.”
Drug companies would save a bundle of money if they did not have to prove to the FDA’s satisfaction that a drug is effective for every medical condition for which they have a shred of evidence. In this case, Caronia did not even mention specific evidence, but merely inferred that his company was “looking at” uses of Xyrem in the treatment of MS, Fibromyalgia, Parkinson’s, etc! Meanwhile, this can put patients at risk whose physicians take such comments as evidence and prescribe a drug as dangerous as Xyrem for off-label indications. As discussed below, FDA has proposed ways for the drug industry to supply off-label information to physicians but only scientifically valid information, not wrapped in the glossed-over language of the typical sales rep.
FDA’s Guidelines for Off-Label Communications
In response to this and previous cases involving off-label communications by the drug industry, FDA has released two important guidance documents: (1) “Good Reprint Practices for the Distribution of Medical Journal Articles and Medical or Scientific Reference Publications on Unapproved New Uses of Approved Drugs and Approved or Cleared Medical Devices” (see here) and (2) “Guidance for Industry Responding to Unsolicited Requests for Off-Label Information About Prescription Drugs and Medical Devices” (see here).
Regarding the distribution of reprints, the FDA has detailed many conditions under which this distribution of “off-label” information would be “allowed.” For example, the articles must be peer-reviewed and come from a journal with an expert editorial board. The article must be accompanied by a prominent warning that the use described is not approved or cleared by thereprints must “not be marked, highlighted, summarized, or characterized by the manufacturer in any way,” “be accompanied by the approved labeling for the drug,” “be distributed separately from information that is promotional in nature,” and “be accompanied by a prominently displayed and permanently affixed statement disclosing any author known to the manufacturer as having a financial interest in the product or manufacturer or who is receiving compensation from the manufacturer, along with the affiliation of the author, to the extent known by the manufacturer, and the nature and amount of any such financial interest of the author or compensation received by the author from the manufacturer,” among others.
FDA abandoned the requirement that drug and device makers must provide the studies to the agency beforehand or promise to seek approval of the discussed use.
Soon after the FDA released the draft guidnace on “Good Reprint Practices,” Pharma Marketing News hosted a survey of readers to get their insights on the issues. Respondents ae asked to indicate how strongly they agreed or disagreed with the following statements regarding the dissemination of off-label drug information by the pharmaceutical industry. Response choices: Strongly DISAGREE, Somewhat DISAGREE, Somewhat AGREE, Strongly AGREE, Neither Agree Nor Disagree. The statements were:
- Under NO circumstances should drug companies be permitted to hand out off-label information — including peer-reviewed journal articles — to physicians and other health care professionals.
- As in the old rule, a drug company should be permitted to hand out such information IF the information is first reviewed by the FDA and the company declares that it intends to submit an application for FDA approval of the off-label use (ie, perform clinical trials).
- FDA is forced to relax the rules because not to do so is an infringement of commercial free-speech rights.
- The new rule will stop pharmaceutical companies from underwriting expensive trials to confirm new drug uses.
- Because the FDA is so slow in assessing drug and device benefits, it is imperative that drug companies be able to hand out medical journal articles so that doctors can learn immediately about life-saving uses.
- Doctors have many other sources of information about off-label use of drugs — the Internet and their own journal subscriptions, for example — and do not require that pharmaceutical companies provide this information to them.
- Since the FDA admitted that it did not really enforce the old requirements, it is also not likely to enforce the NEW requirements (ie, use of peer-reviewed articles only, warning label on articles). Consequently, this gives the drug industry virtual free reign to do off-label promotion.
Results are shown in the following chart: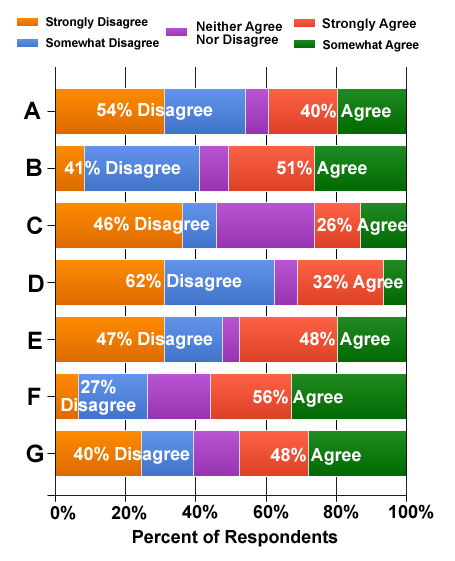
Of interest is the vote on “D”; i.e., “The new rule will stop pharmaceutical companies from underwriting expensive trials to confirm new drug uses.” Sixty-ywo perecent (62%) of all respondents disagreed with this staement whereas only 32% agree. It should be noted that 30% of respondents were employed by FDA-regulated pharmaceutical/device companies and 65% were “very supportive” (42%) or “somewhat supportive” (23%) of the drug industry.
Some comments from respondents include:
“Experience and logic tell us that drug companies will always push limits in the interest of profits. The FDA is simply lowering the bar at the cost of public health.” — Anonymous drug company employee (Somewhat Supportive of the pharma industry)
“Surprise warning letters can still come! I think the whole thing on Free speech is spin, but when we did this practice 10-15 years ago, it helped further medical debate. The question really is who sparks the physician to medical debate and thoughts about innovative therapies? I welcome the reprints back.” — Robert Nauman, Principal, BioPharma Advisors (Very Supportive of the pharma industry)
“I am mostly ‘pro’ this policy, because there are certain disease states that will never be cost-effective to warrant lengthy & expensive trials. If an existing & previously approved medication is found to work in these conditions, then these papers should be able to be handed out. Yes, docs have access to these articles, but how many are actually keeping up with the latest research? If a pharma company does hand out off-label papers, they should be extra stringent about monitoring & reporting AEs related to that off-label use.”— Anonymous drug company employee (Very Supportive of the pharma industry)
“Until companies, regardless of size or income, are forced to comply with regulations executives will continue to find any way possible to encourage off label use. The new guidance appears to almost give them carte blanche to promote off label without taking any risk. As it is many companies pressure employees to support off-label promotional activities; this pressure is not limited to sales & marketing or even medical affairs, it has also drifted into clinical development. The focus on big pharma has actually empowered and emboldended small bio/pharma companies to set up practices that lend an appearane of compliance with no intent to comply. Execs at these companies are extremely sure neither they nor the company will be held accountable since they are acutely aware that, even if a qui tam suit is filed (getting an attorney willing to take a small case is hard), the DOJ/FDA are unlikely to be interested in the cases against ‘small fish’ and others that can fly below the radar.” — Anonymous drug company employee (Somewhat Supportive of the pharma industry)
“The FDA proposal allows very limited distrubution of peer review articles, and puts the FDA — rather than government investigators and others without a clear policy perspective — back in the role as the primary regulator of off-label communication. It’s a careful step.. and thus likely to be beneficial to the public health. Unfortunately, over the past decade these policy decisions have been made more by law enforcement personnel with little real understanding of the underlying policy consequences of their actions. — John Kamp, Executive Director, Coalition for Healthcare Communication (Industry Trade Association known to be supportive of the drug industry)
PMN1111-03
Issue: Vol. 11, No. 11: December 2012



![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-218x150.jpg)




![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




