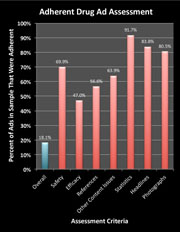
 A study led by Mount Sinai School of Medicine researchers of 192 pharmaceutical advertisements (83 full unique advertisements) in biomedical journals found that only 18 percent (15) were compliant with Food and Drug Administration (FDA) guidelines, and over half failed to quantify serious risks including death. The study was published online on August 17, 2011 in the journal Public Library of Science (PLoS) One.
A study led by Mount Sinai School of Medicine researchers of 192 pharmaceutical advertisements (83 full unique advertisements) in biomedical journals found that only 18 percent (15) were compliant with Food and Drug Administration (FDA) guidelines, and over half failed to quantify serious risks including death. The study was published online on August 17, 2011 in the journal Public Library of Science (PLoS) One.
In short, the authors played the role of DDMAC reviewers and, based on THEIR interpretation of the regulations, determined if the ad under review was non-compliant with FDA “guidelines.
The question is: Why Isn’t the FDA catching citing these ads for violations?
Learn more here: http://bit.ly/nsE2g7
PMN1013-05
Issue: Vol. 10, No. 13
Publication date: 23 August 2011
Find other articles in related Topic Areas:



![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-218x150.jpg)




![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




