Pfizer, Show Us Your Social Media “Playbook” Has It Been Found? What Does It Reveal About Pfizer’s Social Media Implementation Plan?
By John Mack
 Recently, I caught an early New Jersey Transit train to Manhattan to attend BDI’s Social Communications & Healthcare 2011 Case Studies & Roundtables conference. I also moderated two roundtable discussions on Connecting with Healthcare Professionals.
Recently, I caught an early New Jersey Transit train to Manhattan to attend BDI’s Social Communications & Healthcare 2011 Case Studies & Roundtables conference. I also moderated two roundtable discussions on Connecting with Healthcare Professionals.
After the conference, I attended a Tweetup organized by Shwen Ghee (@shwen) at the Birreria (@birreria) rooftop beer garden. Unfortunately, they didn’t let Shwen up — something to do with “regulations.”
Anyway, I enjoyed a couple of beers with Xavier Petit (@xpetit; Shire), Marc Monseau (@MDMonseau; formerly of J&J), Robert Halper (@JNJVideo ; currently the YouTube guy at J&J), I M Wolfman (@imwolfman), and Mark Bard (@digitalhealthco; formerly of Manhattan Research, now at Digital Health Coalition).
Back to the BDI conference. A few presentations were of interest to me and I mentioned them in an on-site interview with Sarah McLellan — the Aussie that Ate the Big Apple. See that that interview embedded below. If you haven’t been interviewed by Sarah, you’re missing a great treat!
John Mack, Pharma Guy, at BDI1 from Zemoga on Vimeo.
As in the past, Ray Kerins (@raykerins), Vice President of Worldwide Communications at Pfizer, made the Keynote presentation, in which he mentioned Pfizer’s “groundbreaking internal Social Media Playbook” that we’ve all been hearing about since last December or January. At that time, Kate Bird (@katebird; Digital Communications Professional for Pfizer), talked about the playbook at a BlogWell conference (see here). Bird explained what is in the Pfizer social playbook: definitions, resource repository, best practices specific to each channel, templates, samples and “watch-outs,” links to all Pfizer channels, and examples of best and “next practices.”
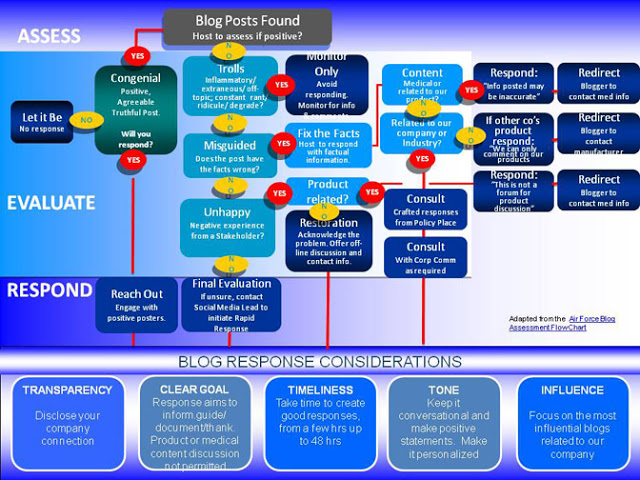
I also hear that the playbook includes this flowchart (from the Canadian branch of Pfizer, see here), which only a process wonk would find useful:
I, of course, want to know if I can get a copy of this “groundbreaking” playbook. So I asked Kerins if there is anything in the playbook that can be made public or if I could get a copy of it. Kerins said: “NO!” and then he backpedaled a bit:
“The playbook and social media policy are constantly evolving. We thought we had it finished last December, and then we realized we didn’t and we went back to update it. So, it’s constantly moving. While I don’t want to say it’s proprietary, I also don’t want to make too big of a deal about it because we call it common sense.”
Then he trailed off about people helping people and said “you’re actually seeing it in action.”
Of course, I am not satisfied with that answer and I don’t see a lot of action in social media as far as Pfizer is concerned. Yeah, it has Twitter accounts and Facebook pages in practically every country. But where’s the social media “meat?” In fact, many of the social media sites attributed to Pfizer in the online Pharma Social Media Wiki (here), such as “MS Voices,” “MS Champions,” and “Addressing Psoriasis” are no longer available. Others, such as the “Our Hemophilia Community” Facebook page are nothing more than drug.com websites for which the playbook has long been out of date. BTW, “Our Hemophilia Community” may soon have to shut down because of Facebook’s new rules that require accepting comments on walls — something that Pfizer does not allow (I guess that’s in the playbook — ie, don’t play with consumers by allowing them to post comments).
Although several people tell me they have actually seen the playbook, no-one can actually give me a copy (citing client confidentiality). Some one sent me “Corporate Policy #407,” which was written in 2008 and covered “Corporate Blogging Policy.” It includes many “groundbreaking” rules such as “Adhere to all Pfizer policies,” “Adhere to all compliance related rules,” and “Do not use discriminatory, harassing, intimidating or offensive language as defined and discussed in Corporate Policy 303, Equal Opportunity.” Basically, it’s telling employees how to make personal posts on blogs.
Corporate Policy #407 cannot be what Kerins was talking about! It does not have anything to do with rules and best practices for official Pfizer social media projects.
Hoping to get hold of the AUTHENTIC Pfizer Social Media Playbook (if it exists), I tweeted this message after the conference:
See below for what may be the precursor to the “playbook.” Also, here’s a great social media playbook from Eloqua, an online marketing company.
[This article originally appeared in Pharma Marketing Blog. Make sure you are reading the source to get the latest comments.]
FDA Intern & the Quest for Social Media Guidelines
 FDA Intern, strange visitor from an Ivy League school who came to FDA with powers and ability far beyond those of FDA Commissioner Margaret A. Hamburg or even former FDA commish Andy von Eschenbach, has actually been on the hunt for Pfizer’s “Palybook” in an attempt to get FDA moving on developing social media guidelines!
FDA Intern, strange visitor from an Ivy League school who came to FDA with powers and ability far beyond those of FDA Commissioner Margaret A. Hamburg or even former FDA commish Andy von Eschenbach, has actually been on the hunt for Pfizer’s “Palybook” in an attempt to get FDA moving on developing social media guidelines!
Let’s join FDA Intern in her latest adventure… 


Is This Pfizer’s “Social Media Playbook?”
I received a policy document from a credible anonymous source that may just be a version of the authentic Pfizer “Social Media Playbook.” You can find the PDF version of this document here.
[Like all good “leakers,” my anonymous source had a motive for leaking this document to me: “I hope the info is helpful to make SM a more acceptable form of employee/customer expression and that large companies shrug off the fear that is currently associated around this platform.” I am not sure this document will help ease those fears or substantiate them. See my analysis below.]
The document includes two sections of interest to me:
- Additional Rules Applicable to Pfizer-Sponsored Social Media, and
- Any Pfizer-Sponsored Social Media Activities Regarding Pfizer Products Are Promotional Materials Subject to Appropriate Review.
[NOTE: This document does NOT include some sections that were described by Kate Bird, Pfizer’s director of digital communications strategy, in her BlogWell presentation; ie, samples and “watch-outs,” links to all Pfizer channels, and examples of best and “next practices.” Bird described the Playbook as being “supplemental” to a longer, more formal social media policy. The document I have may actually be this “formal social media policy” rather than the playbook itself. Regardless, it offers some interesting insights into how Pfizer intends to implement social media programs.]
The first section mentioned above includes this:
“Pfizer-Sponsored external Social Media relating to prescription pharmaceutical products must not permit open fields allowing for real-time publication of user comments or posts [my emphasis]. This prohibition applies to Social Media discussing Pfizer prescription pharmaceutical products as well as Social Media that discusses only disease states and not specific prescription products.”
Although the document states that “the hallmarks of all Social Media are user-generated content and interaction,” Pfizer does not allow Pfizer-Sponsored Social Media projects to use the tools necessary for true interaction… UNLESS moderation can be employed. The document further states:
“Open fields on Pfizer-Sponsored external Social Media relating to prescription pharmaceutical products or disease states may be enabled for moderated comments or posts. If any open fields are enabled, in addition to being screened for adverse events, comments or posts must be moderated and pre-screened prior to publication [my emphasis].”
Unfortunately, pre-screening may not be possible on some social media platforms such as Facebook, especially after it implements its new rules on comments (listen to this podcast “Pharma Facebook Commenting Changes: The Final Story“).
The third section is short, so I will reproduce it all here:
- Any Social Media activity that discusses a Pfizer product, including pipeline products, requires prior approval from the applicable Product Review Committee, Copy Clearance Committee, or comparable review body or process, and must be submitted to regulatory authorities as required by local laws and regulations. For example, in the United States, posts related to Pfizer prescription drugs or sponsored by a Pfizer prescription drug brand team must be approved in accordance with Pfizer Process Governing Review and Approval of United States Product Team Advertising & Promotional Material, Reg. 08-01, and submitted to DDMAC under Form FDA-2253.
[No revelations in the above.]
- Social Media relating to Pfizer prescription pharmaceutical products on any external Social Media:
a) must clearly disclose that the communication is sponsored by Pfizer;
b) must be fully consistent with the approved product labeling and with all applicable Pfizer policies;
c) must give a fair and balanced presentation of the benefits and risks; and
d) should appear only in appropriate media which allow for:
- inclusion of essential information such as the established name and indication as well as
- the important safety information for the product;
- a link to the complete prescribing information; and
- a balanced presentation of safety and effectiveness information with comparable
- prominence and readability.
- Any medium that does not allow for this information to be presented may not be used for Pfizer-sponsored product discussions. Any exceptions to this prohibition must be approved by the applicable Business Unit Counsel and Regulatory Law.
[I highlighted the above in bold because this is important. It means that Pfizer prohibits branded tweets. It does, however, leave room for exceptions.]
- Any team considering any Social Media activities discussing products or pipeline products must consult with their Business Unit Counsel, Regulatory Law, and the Global Privacy Office prior to engaging in such activities.
That’s about it. I look forward to your comments.
[This last section of the above article originally appeared in Pharma Marketing Blog. Make sure you are reading the source to get the latest comments.]
PMN1012-05
Issue: Vol. 10, No. 12
Publication date: 20 July 2011
Find other articles in related Topic Areas:


![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-218x150.jpg)




![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)