Most Credible Bad Ad Complaints are Submitted by Pharma Bad Ad Program is a Success!
By John Mack
According to a Bad Ad Program 2010-2011 Year End Report just issued by the FDA (see here), the program is a success despite the fact that ONLY 125 complaints were deemed worthy of “comprehensive review.” The remaining 203 Bad Ad complaints were presumably filed away in DDMAC’s circular file. Those 125 complaints worthy enough for review lead to 5 enforcement actions.
The agency “does not view the total number of reports, or number of enforcement actions taken as the primary measures for program success,” said FDA in the report. “Instead, FDA’s most important measure of success for this program is the heightened sense of awareness of misleading promotion among HCPs throughout the health care community and the likely useful deterrent this awareness has on drug promoters who might run afoul of regulation absent of such messaging.”
FDA received complaints from three sources: Healthcare Professionals (HCPs), Consumers, and “representatives of regulated industry” (ie, pharma companies ratting out their competitors). The “pharma” group of complaints was the most credible — 58% of those complaints were deemed worthy for “comprehensive review,” whereas 46% of HCP complaints and only 21% of consumer complaints made the cut (see chart below; click for enlarged view).
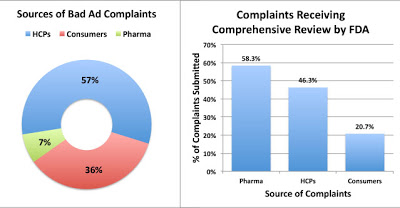
FDA says that the quality of complaints submitted by HCPs “demonstrates a relatively strong level of knowledge in the medical community about what constitutes misleading promotion.” Since pharma’s complaints were even MORE credible than those from HCPs, FDA should have said it “demonstrates a relatively strong level of knowledge in the PHARMA community about what constitutes misleading promotion.”
It’s not clear, however, if “representatives of regulated industry” include HCPs employed by pharma companies. At least one recipient of an FDA Bad Ad enforcement letter claimed the complaint came from such as source (see “Competitors Are “Best Source” of Complaints About Promotions Received by FDA“).
[This article originally appeared in Pharma Marketing Blog. Make sure you are reading the source to get the latest comments.]
FDA’s Bad Ad Program is “Phoniest Thing Ever!”
So says Jerry Roth, president of Sanford, Fla.-based Hill Dermaceuticals. His company received the ONLY letter from the FDA as as a result of a complaint received via DDMAC’s (Division of Drug Marketing, Advertising, and Communication) Bad Ad Program (see here).
According to Advertising Age, FDA received 239 complaints to the Bad Ad program from its launch in May through January of this year. “Of these complaints, 129 (54%) have come from health-care providers, 73 (31%) from consumers, and the remainder from other sources. Of the 239 complaints, approximately 135 were deemed to have sufficient merit for further investigation, with approximately 50% falling within DDMAC oversight and the rest being referred to other centers within FDA.”
According to DrugWonk’s Peter Pitt, “At best this effort isn’t, um, cost effective” (see here).
I myself submitted a compliant to the FDA regarding this Lyrica DTC Ad. I didn’t get any response from the FDA due to this “technical glitch” in the auto-response to submissions to its BadAd program. The belated response came from “Bob Dean,” the lead consumer safety officer for DDMAC. Dean worked as Senior Specialty Sales Representative at Johnson and Johnson for five years and as a Sales representative at UCB for 2 years (see his LinkedIn profile).
Mr Roth claims DDMAC acted on a complaint filed by “a doctor for another [competing] company,” which is interesting. The FDA solicited complaints from doctors because “prescriber[s] can play an important role in ensuring that prescription drug advertising and promotion is truthful by recognizing and reporting misleading drug advertising and promotion.” But because so many — perhaps up to 80% of — “prescribers” are employed directly or indirectly by pharmaceutical companies, the Bad Ad program is open to criticisms similar to Mr Roth’s.
Roth also said “I sell my products at around $50 a prescription when the average prescription is near $200. So you figure out why they came after me.” Given Mr. Dean’s background in Big Pharma, I can feel Mr. Roth’s pain!
The letter Hill Dermaceuticals received cited that certain pages on the company’s website were “false or misleading because they omit and minimize the risks associated with the use of Derma-Smoothe Body Oil, overstate its efficacy, present unsubstantiated superiority claims, broaden and inadequately communicate the indication, and present unsubstantiated claims for the drug product.” This is interesting because that’s EXACTLY what I said about Pfizer’s Lyrica DTC Ad!
Poll: Do you think FDA’s Bad Ad Program is:IneffectiveBiasedNot worth the costAll of the aboveNone of the above
PMN1011-03
Issue: Vol. 10, No. 11
Publication date: 21 June 2011
Word Count: n/a
Find other articles in related Topic Areas:


![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-218x150.jpg)




![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




