Pharma Industry News Update: 15 Dec 2017
Grading Gottlieb & PhRMA
From Raves to Meh!
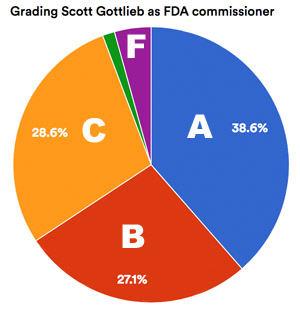
[From www.statnews.com] Food and Drug Administration Commissioner Scott Gottlieb earned high marks from [a STAT Plus] survey respondents.
One respondent explained why Gottlieb deserved an F: “I see no vision or mission except keep Trump happy. Good luck.”
But the vast majority sang his praises.
The trade group PhRMA has tried to reframe the drug price debate with its “Go Boldly” campaign, paid for by a hike in membership fees. In decidedly lukewarm reviews, more than a third of respondents gave that initiative a modest 2 on a scale of 1 to 5 (with 5 being a rave). Roughly a quarter split between 1 (the worst) and 3 (meh).
Further Reading:
Re Gottlieb:
- Dr. Scott Gottlieb’s Plan to “Get Things Done” at #FDA is Approved by #Pharma; http://sco.lt/7iW5TN
- Does Pharma Really Want to Abandon the “Gold Standard” of the FDA Approval Process?; http://sco.lt/74r19d
- Pharma Scientists Concerned About Gottlieb’s Industry Ties; http://sco.lt/8jOq0n
Re PhRMA:
- He Boldly Stars in @PhRMA’s Multi-Million $ Ad Campaign, But Has No Money to Pay His Rent; http://sco.lt/5BIFgP
Gottlieb Proposes Even Faster Track Approvals for Medical Devices
This is Why Industry Loves Him!

[From www.reuters.com] Commissioner Scott Gottlieb’s proposals make good on President Donald Trump’s promise to cut regulations and sparked concern from public health advocates who fear the moves will harm patients. Already dozens of devices are recalled each year.
Gottlieb’s proposal would offer an alternative route to market for certain companies which do not meet the criteria for clearance under the agency’s existing fast track route, known as the 510(k) pathway.
His new proposal would dispense with the need for a predicate [an equivalent existing device] and offer the option of using a benchmark consisting of a set of performance standards or guidance documents.
Dr. Rita Redberg, a cardiologist at the University of California San Francisco and editor of JAMA Internal Medicine, said shifting safety monitoring from the pre-approval to the post-market setting would essentially turn patients into guinea pigs.
“When we use devices on patients without clinical studies first, the patients effectively become the trial and the insurers become the funder of the device trial,” she said.
PharmaGuy’s Insights:
Here’s the fly in this ointment: Who advises the FDA how to “modernize” the performance benchmarks? I suspect that the FDA will depend upon outside experts with ties to the industry!
Further Reading:
- Streamlined FDA Reviews Fail to Catch Potentially Deadly Risks Due to Faulty Medical Device Software; http://sco.lt/7TlGgT
- FDA Does Not Do Its Job viz-a-viz Medical Devices, Says Patient Advocate; http://sco.lt/5PrLcH
Health Organizations Attempt to Rein In “Wild West” of mHealth Apps
Xcertia Collaboration Issues Preliminary Guidelines

[From www.prweb.com] The early version of the guidelines assesses the quality, safety and effectiveness of mobile health apps in four key areas:
- Operability – to assess whether a mobile health app installs, loads, and runs in a manner that provides a reasonable user experience.
- Privacy – to assess whether a mobile health app protects the user’s information, including Protected Health Information in full compliance with all applicable laws, rules and regulations.
- Security – to assess if the application is protected from external threats.
- Content – to assess whether the information provided in the mobile health app is current and accurate.
The guidelines are backed by Xcertia’s growing collaboration of 30+ members, including its founders, the American Heart Association, the American Medical Association (AMA), the DHX Group, and the Healthcare Information and Management Systems Society (HIMSS).
Further Reading:
- Patient Activists Demand Higher Quality Mobile Health Apps; http://bit.ly/pmn130701pdf
- Reigning in the “Wild West” of Mobile Health Apps; http://bit.ly/mobcat-pmn1202-01
- EU’s mHealth “Moon Shot:” Validate Data Collected by Apps!; http://sco.lt/6NSVZh
- AMA Adopts Principles to Promote Safe and Effective Mobile Health Applications; http://sco.lt/7PdiWf
- MIT Hackers (@MITHackMed) to “Certify” Consumer mHealth Apps. WHAAA?!; http://sco.lt/5CSwiX
- Another Mobile Health App “Certification” Program. Will It Succeed or #FAIL?; http://sco.lt/7pVpyb
- Pharma Calls for a New Regulatory Framework for Digital Health Apps; http://sco.lt/87u3ft
- Is IMS Health’s Mobile Health App “Certification” Program Doomed to #FAIL?; http://bit.ly/mobileranking








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)