Pharma Industry News Update: 8 November 2016
Featured Survey 
Direct-to-Consumer Off-Label Drug Promotion SurveyThis survey solicits your opinion possible FDA future decisions regarding off-label drug promotion to patient and consumer audiences. CLICK HERE to take the survey.
![]()
 Meet PharmaGuy at FDA Public Hearing on Off-Label Promotion on November 9, 2016
Meet PharmaGuy at FDA Public Hearing on Off-Label Promotion on November 9, 2016
PharmaGuy will be speaking at FDA’s Part 15 hearing on “Manufacturer Communications Regarding Unapproved Uses of Approved or Cleared Medical Products” on 9 November, 2016.
The purpose of this meeting is to obtain “input on issues” related to off-label product communications about by pharmaceutical and medical device companies (see here).
Pharmaguy will present some preliminary results from two surveys regarding direct-to-consumer off-label drug promotion: (1) an online survey hosted by Pharma Marketing News and primarily focused on pharma executive participants (see preliminary results here) and (2) an inVibe voice-response survey of patients, patient advocates, and caregivers sponsored by Pharma Marketing News.
Click here for the list of speakers.
Related articles:
- In a Surprise Move, FDA Will Hold a Public Meeting to Review Off-label Marketing
- FDA May Have No Choice But to Allow Direct-to-Consumer Off-Label Drug Promotion
- FDA Public Hearing: Pharma Communications Regarding Unapproved Uses of Drugs
- @zdunnhealth in Favor of Direct-to-Consumer Off-Label “Communications” to Consumers as Long as Not “Promotional”
- Comments from Center for Medicine in the Public Interest on Off-Label Drug promotion
- Kamp to Urge FDA to Provide Clear Guidance on Off-label Communication at #FDAofflabel Public Hearing
- Preliminary Results of Direct-to-Consumer Off-Label Drug Promotion Survey Submitted to FDA
Review: The Use of Social Media in Recruitment for Medical Research Studies
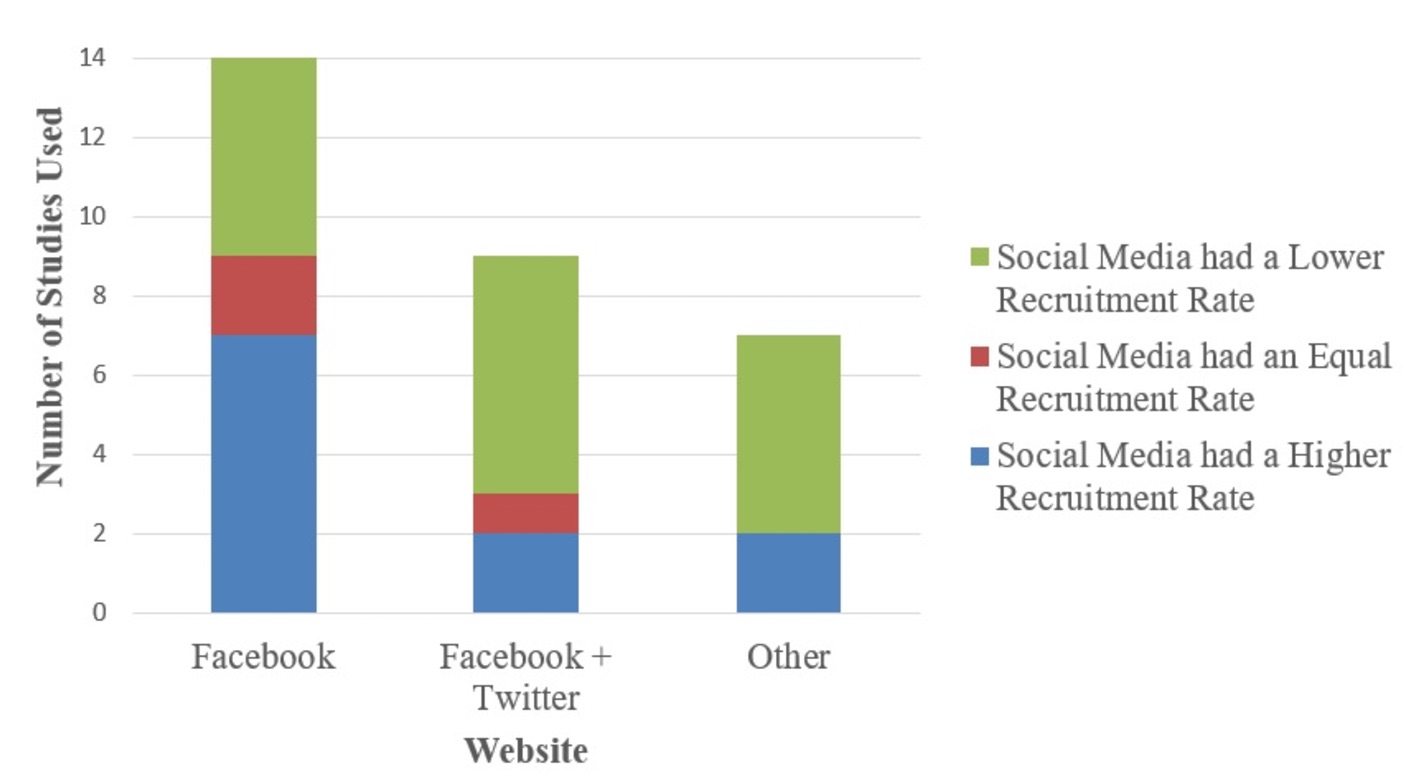
Background: Recruiting an adequate number of participants into medical research studies is challenging for many researchers. Over the past 10 years, the use of social media websites has increased in the general population. Consequently, social media websites are a new, powerful method for recruiting participants into such studies.
Objective: The objective was to answer the following questions: (1) Is the use of social media more effective at research participant recruitment than traditional methods? (2) Does social media recruit a sample of research participants comparable to that recruited via other methods? (3) Is social media more cost-effective at research participant recruitment than traditional methods?
Related articles:
- Clinical Trials Need More Subjects
- Direct patient engagement through social media speeds recruitment to cancer research study
- PatientsLikeMe Launches New Services That Make Patients Partners in Medical Research
- Using Social Media to Attract Study Participants
- How Novartis is transforming clinical trials with social media
- How to Get 11,000 Clinical Trial Participants Signed Up in One Day!
- Social Media and Clinical Trial Recruitment
![]()
 Mount Sinai Platform Curates Digital Health Apps
Mount Sinai Platform Curates Digital Health Apps
Providers interested in prescribing digital apps to help patients monitor and communicate about their health are sometimes overwhelmed by the sheer volume of mobile apps on the market. There are more than 245,000 medical apps available.
To address this issue, researchers in the Sinai App Lab at the Icahn School of Medicine at Mount Sinai Health System have developed RxUniverse, an enterprise-wide tool that curates apps and enables physicians to digitally prescribe evidence-based apps to patients at the point of care.
More here…
Related articles:
- Doctors Say They Recommend mHealth Apps to Patients, But Patients Say They Don’t!
- AMA Survey Finds That Many Physicians Are Enthusiastic About Digital Health Tools, But Few Currently Use Them
- Doctors Still Don’t Trust mHealth Apps
- MIT Hackers (@MITHackMed) to “Certify” Consumer mHealth Apps. WHAAA?!
- High mHealth App Ratings Do Not Correlate With Actual Quality
![]()
 Drugmakers, Facing Pricing Criticism, Sell Cures in New Ads
Drugmakers, Facing Pricing Criticism, Sell Cures in New Ads
The spot starts dramatically with a 30-something-year-old man explaining, “Before it became a medicine, it was an idea, an inspiration, a wild what-if.” The scene duly set, the ad goes on to tell the story of the scientists at Pfizer who discovered, fought for, and brought to market the pill he had just shaken into his hand. “It became a medicine so someone who could not be cured could be — me,” he adds, swinging his son into his arms.
This Pfizer ad, Driven to Discover the Cure (read Pfizer Launches an Ad Campaign to Improve Its Rep), is one of several recent campaigns that seek to reinforce the idea that years of hard work — and heart — go into the development of new medicines. The campaigns present an image of the industry that clashes with the prevalent one – big bad pharma reaping profits hand over fist at the expense of a sick and underinformed public.
Many of these new campaigns focus on the parallel ideas of innovation and cures, despite the fact only a single class of drugs approved by the FDA in the last five years — a class led by Gilead Sciences’ hepatitis-C drug Sovaldi — is considered a cure for a disease or condition.
More here…
Related articles:








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)