Pharma Industry News Update: 24 Oct 2017
Few Patient Groups DON’T Take Pharma $![]()
 Look at Stands Not Taken
Look at Stands Not Taken
[From www.bna.com] Only a handful out of 7,685 health advocacy groups in the U.S. are completely independent of pharmaceutical industry money, according to a list the group PharmedOut released Oct. 13. PharmedOut is a Georgetown University Medical Center project that advances evidence-based prescribing and educates health-care professionals about pharmaceutical marketing practices.
And industry funding of patient groups, including websites and informational materials, is often not apparent to the average consumer, which could mislead consumers into believing they’re getting unbiased health advice.
“Industry funding is often not disclosed on websites or informational materials or is hidden,” PharmedOut Director Adriane Fugh-Berman told a reporter in an Oct. 16 phone call. “Funding and sponsorship is often very subtle and difficult to identify,” she said.
Groups that accept industry funding are affected by that money, regardless of whether they think they are, she said. “Look at the stands taken and not taken,” she said. “For example, where is the anger and outrage about drug costs?” However, read:
Further Reading:
- 93% of Patient Advocacy Groups Included in FDA Funding Discussions Receive $ from Pharma
- More Than Two-thirds of Patient Advocacy Groups Receive Industry Funding
- Pharma “Patient Centricity” Aids & Abets the Opioid Epidemic
- How a #pharma Funded “Grassroots” Patient Advocacy Campaign Changed FDA’s Approval Process
- Patient Advocacy Groups with Funding & Form Letter from @PhRMA Oppose Nevada Legislation
- Pharma Lines Up Patient Groups to Fight for PDUFA Boondoggle
CX and Pharma’s Search for Excellence
Whilst many other industries such as finance and retail are locked into the effect a positive customer experience can have on sales, loyalty and advocacy, pharma is still lagging behind.
In eyeforpharma’s new exclusive whitepaper “CX and Pharma’s Search for Excellence” we tackle the factors needed to ensure you are continually delivering a great experience, including:
- Industry Case Studies from TEVA, Pfizer, Merck, BMS, and AstraZeneca showing you how your peers are embracing customer experience
- How other industries embrace CX, and what can be learnt from companies like Netflix, Starbuck and the finance industry
- The 8 golden rules to getting to grips with your customer
Download this paper here.
Sponsored by FC Business Intelligence Ltd.
Is FDA Set for Another Banner Year of Approvals?
It Appears So!
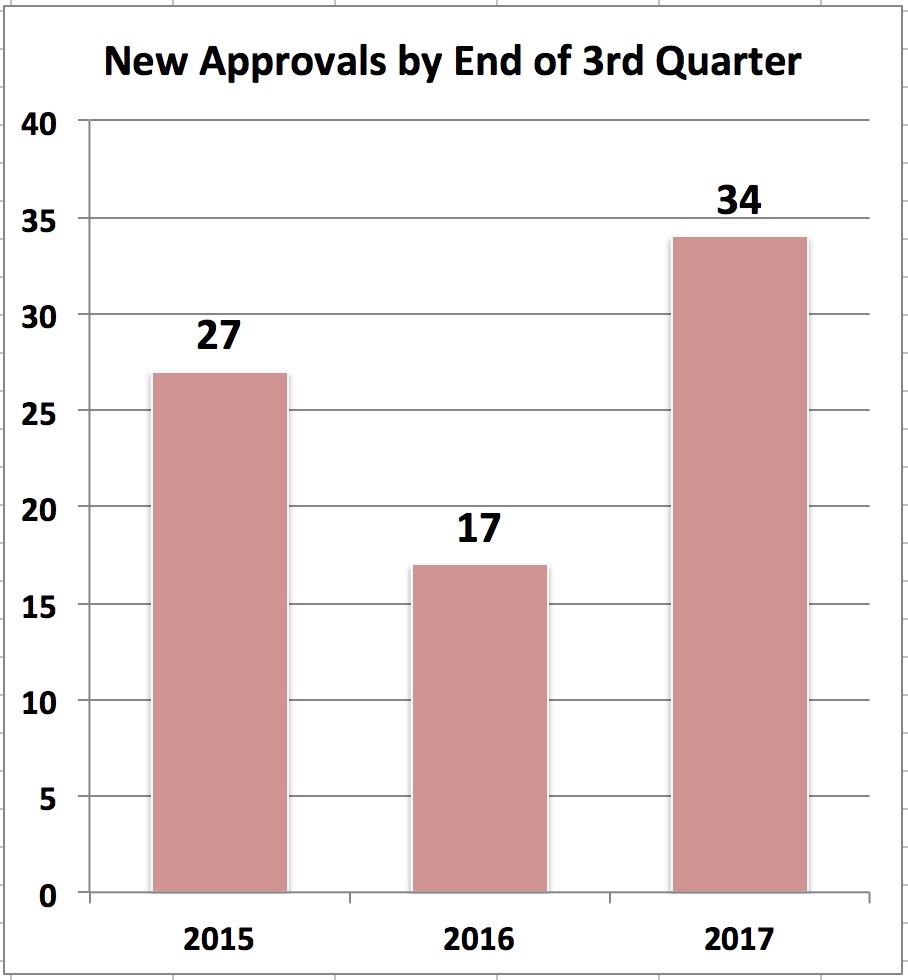
[From eyeonfda.com] Comparing the first three quarters of the last three years, one can see that 2017 is on track to be an important year for approvals and if it keeps up at the current pace, could overtake 2015 in bringing new medicines out of the pipeline.
As one can readily see, by the end of the third quarter, new approvals were outstripping 2015 – 34 in 2017 compared to 27 in 2016 and twice the number of 17 just last year.
Further Reading:
- FDA Green Lights More Novel Drug Approvals
- Drugs Approved by #FDA So Far in 2017 are Mostly Targeted to Smaller Patient Populations
- Is FDA Too Slow to Approve New Drugs?
- FDA to Use Computer Modeling to Speed Up the Drug Approval Process
Why Do We Need Drug Rebates, Anyway?![]()
 Good Question Senator!
Good Question Senator!
[From www.statnews.com] Sen. Lamar Alexander has a question: why do we have drug rebates, anyway?
“Why do we need rebates?” the Tennessee Republican asked a panel of pharmaceutical industry representatives at a Senate committee hearing. The Health, Education, Labor, and Pensions committee met Tuesday morning for the second of three hearings on drug pricing, and heard testimony from five interest groups representing companies that play different roles in getting medicines to patients.
“Why don’t we just get rid of rebates and let you negotiate directly with manufactures, take that $100 billion a year, and just reduce the list price?”
Alexander turned to the drug manufacturers at the hearing, represented by Lori Reilly, executive vice president for policy, research, and membership at the trade group Pharmaceutical Research and Manufacturers of America.
“Would you like to eliminate rebates?” Alexander asked.
Further Reading:










![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




