Pharma Industry News Update: 3 Oct 2017
The Pharma Marketing Salary Roller Coaster
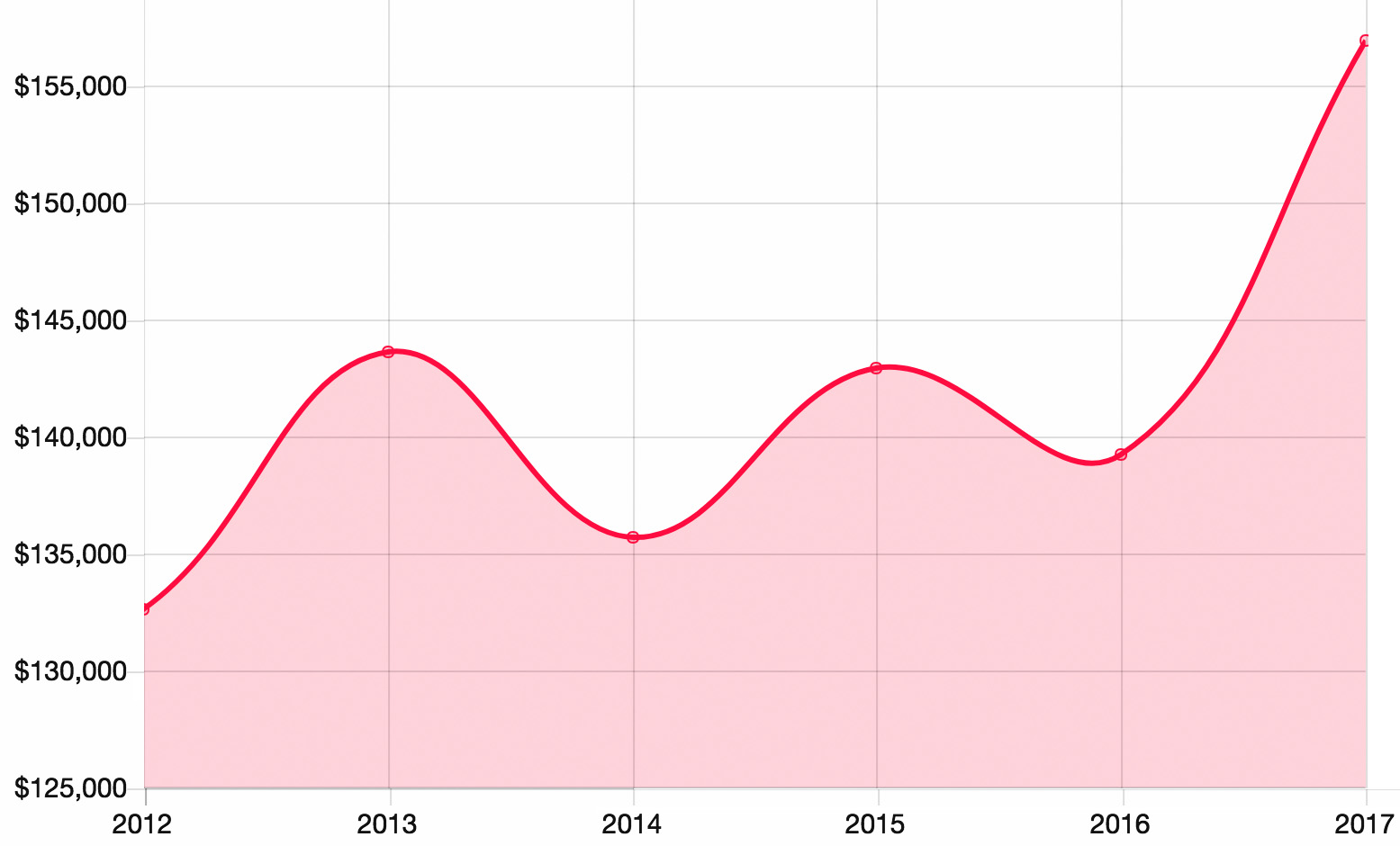
Ready for the Next Big Dip?
[From www.mmm-online.com] Welcome to the 31st MM&M Career and Salary Survey, which brings tidings both welcome (salaries are up) and somewhat less so (the gender gap hasn’t narrowed much). Buckle up – there’s lots of ground to cover.
As with any survey of this nature, the headline data are always the ones related to salary. Based on conversations with marketing higher-ups at pharma companies and agency leaders, we expected to see some salary inflation from a year ago. What we didn’t expect was the sizable percentage jump…
Further Reading:
- Pharma Marketers’ Salaries Dropped 2.6% in 2016 But Women Catching Up – Slowly!
- #Pharma Senior Marketers Paid More Than Banking Marketers – Digital Not So Much
![]()
 Academic Research Goes Where No Pharma Was Willing to Go…
Academic Research Goes Where No Pharma Was Willing to Go…
Develops a Universal Flu Vaccine
[From www.pmlive.com] A UK company has started trialling a new universal influenza vaccine that would avoid the annual scramble to guess the most likely strains to be circulating in the following flu season.
The vaccine – developed by University of Oxford spin-out Vaccitech – will be tested in around 500 National Health Service (NHS) patients in a study supported by the National Institute for Health Research (NIHR). It is thought to be the first trial of such a vaccine and will extend over the 2017-18 and 2018-19 flu seasons.
The need for a more effective flu vaccine was starkly revealed last winter. The recommended vaccine was around 40% effective overall, but hardly provided protection at all to the over-65s who are most at risk of severe complications and death from the infection, despite being a good match for circulating strains of the virus.
Further Reading:
- Your Next Flu Vaccination May Not Be as Effective as You Think
- Flu Shot Doesn’t Work as Well as #pharma Clinical Data Suggests
- Everything You Wanted to Know About Vaccine Marketing, PR, Earned Media, Lobbying, and “Anti-Vaxxers”
- Adults Only Really Catch the Flu About Twice a Decade, Study Suggests: Another Good Reason Not to Get Flu Vaccine
PharmaGuy’s Insight:
I was involved tangentially in research to develop a universal flu vaccine way back in the early 80s (see “Pharma Needs to Step Up & Help Develop a Universal Flu Vaccine“). The image shown here is of a molecular model I built of the core protein of the flu virus.
![]()
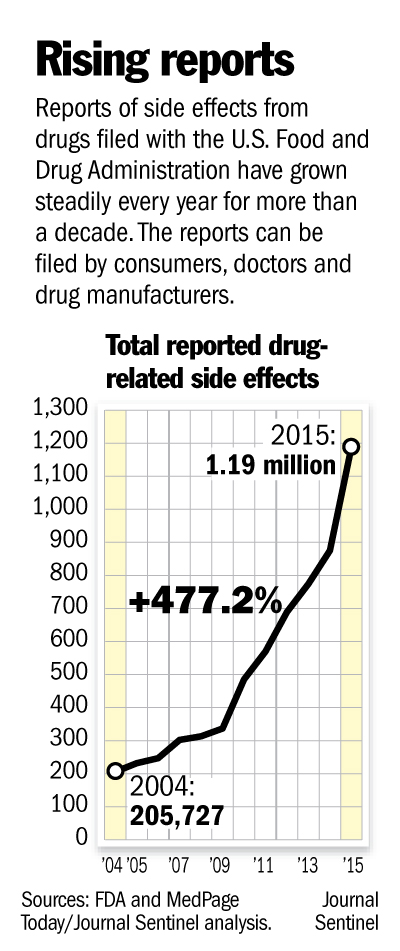 FDA Makes Side-Effect Database Searchable
FDA Makes Side-Effect Database Searchable
Develops a Universal Flu Vaccine
[From www.bloomberg.com] The U.S. Food and Drug Administration improved its online database of reports about side effects filed by patients and doctors, making it easily searchable by product, patient age, type of side effect or year it occurred.
It means that, for the first time, the public will have access to data it can make sense of. Previously, inquiring minds needed to know a little about coding to figure out the FDA’s adverse-reaction database. And even then, they had to file a Freedom of Information request with the government to get the actual reports and check the information’s accuracy.
Further Reading:
- Another Aspect of Patient Power: Making It Easier to Report Adverse Events!
- Institute for Safe Medicines Practices Calls for a Full-scale Modernization of FDA’s Adverse Event Reporting System
- So Far, FDA’s Sentinel Drug Safety Monitoring Initiative Has Not Delivered as Hoped
- OpEd: FDA’s Antiquated Drug Safety Program is “Obscene”








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




