Pharma Industry News Update: 18 August 2017
Patient Participation in Health Care Conference Social Media Discussions
Is It a Good Idea?
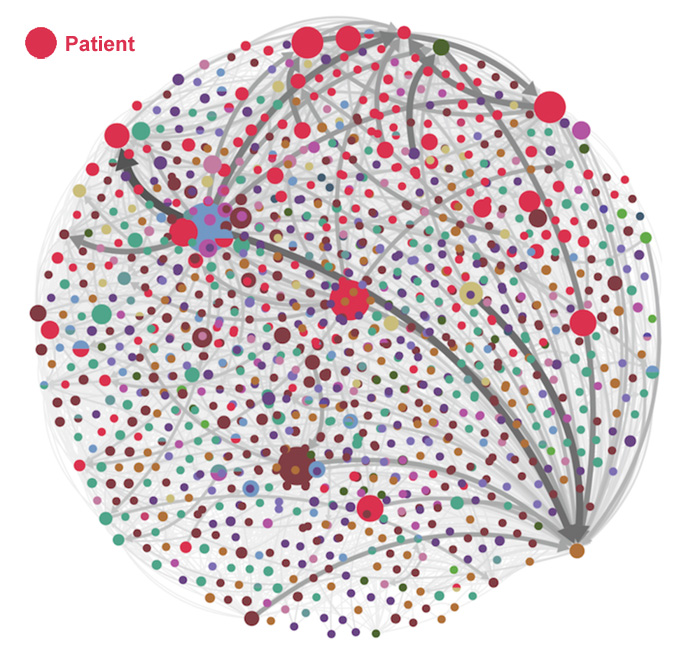
[From www.jmir.org] Objective: The purpose of this study is to examine the impact engaged patients bring to health care conference social media information flow and how they expand dissemination and distribution of tweets compared to other health care conference stakeholders such as physicians and researchers.
Conclusions: Although engaged patients are powerful accelerators of information flow, expanders of tweet propagation, and greatly deepen engagement in conversation of tweets on social media of health care conferences compared to physicians, they represent only 1.4% of the stakeholder mix of the top 100 influencers in the conversation. Health care conferences that fail to engage patients in their proceedings may risk limiting their engagement with the public, disseminating scientific information to a narrow community and slowing flow of information across social media channels.
More about the study’s methods and results…
Further Reading:
Tumblr Low on Pharma’s Most Popular Social Media Platforms List
But Some Pharma Social Media Consultants Disagree
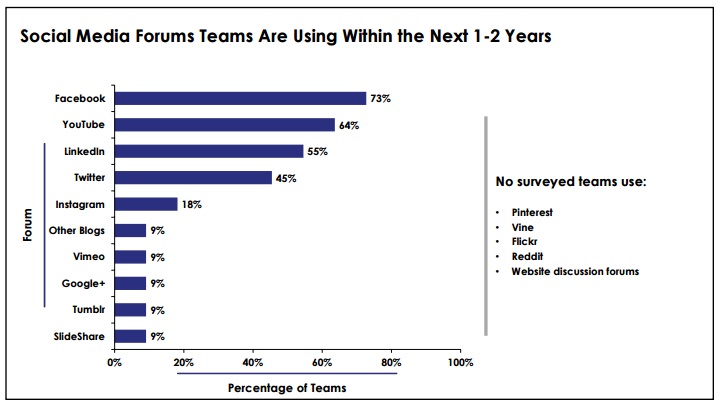
[From www.marketwired.com] A new study of pharmaceutical marketing teams found that the most popular social media forum groups plan to use in the next one to two years is Facebook (73%), according to data published by business intelligence firm Cutting Edge Information.
Data found in the study, Pharmaceutical Marketing: Reevaluate Digital Trends and Metrics for Social Media and Mobile Success, which was published less than a month ago, revealed that YouTube (64%) and LinkedIn (55%) are also common for surveyed life science marketing teams, with Twitter (45%) at a more distant fourth.
Not as popular, but still commonly used social networking sites include Instagram and Tumblr, with 18% and 9% of pharmaceutical marketing teams utilizing them, respectively.
But some consultants say Tumblr deserves more respect from pharma. Read Pharma Tumbles for Tumblr. It’s Not Your Grandfather’s Social Media!
Further Reading:
![]()
 50% of Fast-Track Approved Drugs Have Not Been Proved Effective After 3 Years on the Market!
50% of Fast-Track Approved Drugs Have Not Been Proved Effective After 3 Years on the Market!
Most Are Cancer Drugs!
[From www.statnews.com] The FDA, in an effort to bring promising new therapies to patients as quickly as possible, has introduced a spate of shortcuts to speed up the approval process. Those programs are working as intended, new research finds, but drug companies are often loath to fulfill their obligations.
The big idea behind the FDA’s accelerated drug approval program is that regulators will OK a promising drug based on clues that it will improve patient lives, so long as pharma companies later carry out larger trials to confirm those hints of efficacy. But looking at four years of data, a team of researchers found that only 50 percent of those trials actually took place within three years of approval.
Furthermore, 44 percent of such trials were not the placebo-controlled variety considered to be the gold standard but rather relied on the same surrogate measures used to win a quick approval, leaving each drug’s true value unclear. This was particularly striking for cancer drugs, accounting for 80 percent of studied approvals, which were cleared based on how well they shrank tumors, not how long they kept patients alive.
Pharmaguy’s Insights:
According a Research Letter published in the July 10, 2013, issue of JAMA. The authors of the study found that NONE (zero) of the 865 studies under FDAAA jurisdiction from 2008 through 2011 have been completed. Of the 387 studies mandated in 2011, 271 (70%) have not even begun.
Further Reading:








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)