Pharma Industry News Update: 28 July 2017
![]()
 Celgene Settles $280 Million Fraud Suit
Celgene Settles $280 Million Fraud Suit
Promoted Cancer Drugs Off-Label
[From www.nytimes.com] The pharmaceutical company Celgene has agreed to pay $280 million to settle claims that it marketed the cancer drugs Thalomid and Revlimid for unapproved uses, the company said on Tuesday.
Under the terms of the settlement, which resulted from a lawsuit filed by a whistle-blower – a former sales representative at Celgene – the company will pay $259.3 million to the United States and $20.7 million to 28 states and the District of Columbia.
Cancer drugs are seen as more difficult to pursue in so-called off-label marketing cases in part because oncologists often prescribe drugs for unapproved uses in an effort to combat a deadly and still mysterious disease.
“The company got the idea that it could be fast and loose with what it was saying about its drug because it was selling to cancer patients who might be in need,” Mr. Guttman said. “At the end of the day, what this is about is that even when you’re on life’s edge,” he added, a company “can’t break the law by off-label marketing a drug.”
Further Reading:
- A Pharma Marketing “Bait-and-Switch” Scheme: Sales Reps Disguised as Medical Science Liaisons
- Thalidomide Offsprings Yield Blockbuster Profits for Celgene Aided by Off-Label Promotion
- Off-label Promotion by the Numbers: Settlements, Warnings, Fines, % Prescribed
- Allowing Pharma Sales Reps to Discuss Off-Label Drug Use Would Make Them More Helpful, Says KevinMD
Sponsor Message Better Align Sales and Marketing to Achieve Commercial Success
PharmaForce is the go-to event for marketing, sales and strategy leaders from the leading pharmaceutical companies to take on key industry challenges in sales, policy, patient-centricity and more.
Here’s what makes PharmaForce unlike any other event in Pharma:
- Designed to Help You Connect. 96% of attendees agree PharmaForce is the best networking they’ve ever experienced.
- Align Your Strategy. 40% of sessions cover both a marketing and sales perspective.
- A Senior Focus. Be inspired hearing from and meeting with 50+ of the biggest names in the industry. See who’s speaking by downloading the agenda.
- Have a Blast in Austin! Get ready for great barbecue, craft cocktails, and surprises you’ll have to stay tuned for.
Join 200+ of your peers at PharmaForce September 18-20, 2017 at the Hilton in Austin, TX.
You’ll go back to the office with tons of notes, new ideas, and plans for the future. And you’ll have a great time, with a strong focus on small group discussions and structured networking (that’s actually fun), you’ll have many chances to meet new friends and explore Austin!
Download the agenda here to see the full list of topics covered at the event!
Register now and save 20% with code PF17PMN.
Sponsored by Worldwide Business Research.
FDA is Looking for a Few Good Digital Health Product Developers
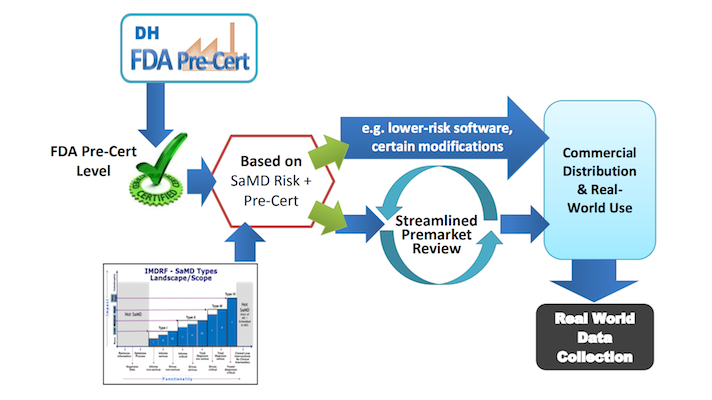
Who Will Be the Lucky Nine?
[From blogs.fda.gov] FDA announced, as part of its broader Medical Innovation Access Plan, a new component focused on digital health innovation – the formal launch of its Pre-Cert for Software Pilot Program.
This new program embraces the principle that digital health technologies can have significant benefits to patients’ lives and to our healthcare system by facilitating prevention, treatment, and diagnosis; and by helping consumers manage chronic conditions outside of traditional healthcare settings.
At the same time FDA announced this pilot, FDA’s Center for Devices and Radiological Health (CDRH) is publishing its Digital Health Innovation Action Plan to provide details and timelines for FDA’s integrated approach to digital health technology and the implementation of the 21st Century Cures Act. FDA is telling consumers and the digital health industry how it will establish clear and consistent expectations for the products FDA regulates.
FDA designed the new digital health pilot program to include up to nine software firms of various sizes. Initial participants in this new pilot will range from small startups to large companies that develop both high- and low-risk software products that are devices.
Learn how to qualify for the program…
Further Reading:
![]()
 FDA Making “PEACe” with Patients
FDA Making “PEACe” with Patients
Schedules First Ever Patient Engagement Advisory Committee Event
[From www.eyeonfda.com] This week marks a new milestone in the arc of progress that has been patient involvement since that time. FDA announced in a blog posting on FDA Voice, and in a Federal Register notice, that the inaugural meeting of the Patient Engagement Advisory Committee (PEAC) will be held in October. The first meeting will take place on October 11 and 12 and the agenda is focused on getting input into getting patient input into medical device trials.
The current roster of the committee has 9 members, two of whom are from disease-specific organizations – the American Association of Kidney Patients and the Arthritis Foundation. Other member slots represent individuals who work in the area of patient engagement. At least one person appears to be experienced in minority outreach, but unlike other FDA Advisory Committees, the CV’s of the members are not yet linked to their names on the current roster listing.
The launch of the committee is an important step, but it is important to note that it is one step in a much longer journey.
Further Reading:









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




