Pharma Industry News Update: 12 May 2017
Big Pharma Struggles to Distance Itself from “Price Gouging” Small Pharma The drug industry is reeling from criticism about drug prices coming from all sides and especially from the president of the United States. First it tried public relations and CEO speaking tours. When that didn’t work it took more drastic action.
PhRMA’s Big Small-Company Purge:
22 Companies Stripped of Their Membership Status

[From www.axios.com] PhRMA, the pharmaceutical industry’s primary lobbying group, announced changes to its membership structure that include stricter membership requirements and the elimination of its “associate” category. As a result, 22 companies lost their membership or associate membership status. The new requirements, based on a three-year average:
- At least $200 million per year spent on research
- Research expenditures equal to at least 10% of global sales
Former members, include:
- AMAG Pharmaceuticals, Inc.
- Horizon Pharma plc
- Jazz Pharmaceuticals plc
- Mallinckrodt Pharmaceuticals
- ACADIA Pharmaceuticals Inc.
- BioMarin Pharmaceutical Inc.
- CSL Behring, LLC
- Ferring Pharmaceuticals Inc.
- Marathon Pharmaceuticals, LLC
- Shionogi Inc.
- Sucampo Pharmaceuticals, Inc.
Further Reading:
It was only after meeting with Trump that PhRMA got religion and started to reconsider whom it should accept as members.
- PhRMA Embarrassed by Marathon is Forced to Review Membership Criteria. Is a Purge in the Cards?
- PhRMA Offers Up Marathon Pharmaceuticals as “Sacrificial Lamb” to Trump
J & J’s Legal Woes Continue to Escalate as DOJ Targets Its Rx Drug Marketing Practices

[From www.fiercepharma.com] As if Johnson & Johnson didn’t have enough legal woes already, with the mounting number of cases claiming a link between its talcum powder and ovarian cancer (read J&J Bites the Talc-powder Dust in Another Trial – Ordered to Pay $110 Million), the pharma giant now faces a fresh round of investigations into its marketing practices.
In a quarterly filing with the Securities and Exchange Commission, J&J disclosed new investigations by the U.S. Justice Department and the U.S. Attorney’s Office in Massachusetts. The probes target arthritis drugs Remicade and Simponi, hepatitis C treatment Olysio and psoriasis drug Stelara.
Most recently, in April, J&J was subpoenaed by the Massachusetts district court, which is seeking documents related to copayment-support programs the company is offering for Olysio, Simponi and Stelara, according to the SEC filing. Investigators are seeking information about how J&J reports the prices of those products to the Centers for Medicare & Medicaid Services and how it discloses rebate payments to the state’s Medicaid agencies.
Further Reading:
- Giving J&J an Ethics Prize is Like Giving Strom Thurmond a Civil Rights Award
- Johnson & Johnson Guilty Again! Ordered to Pay $1 Billion in Putative Damages
- The $70 Million Breast Job: That’s What J&J Must Pay to Male Teen Who Took Risperdal
- How Gorsky Drove 46% – 66% of Risperdal Sales for Off-Label Use
- America’s Most Admired Lawbreaker
- J&J Pleads Guilty for Knowingly Selling Tainted Children’s Tylenol
Despite all that, J&J’s earnings rose 18.7% in 2014! (see story here).
Nearly One-Third of New Drugs Are Affected by a Postmarket Safety Event
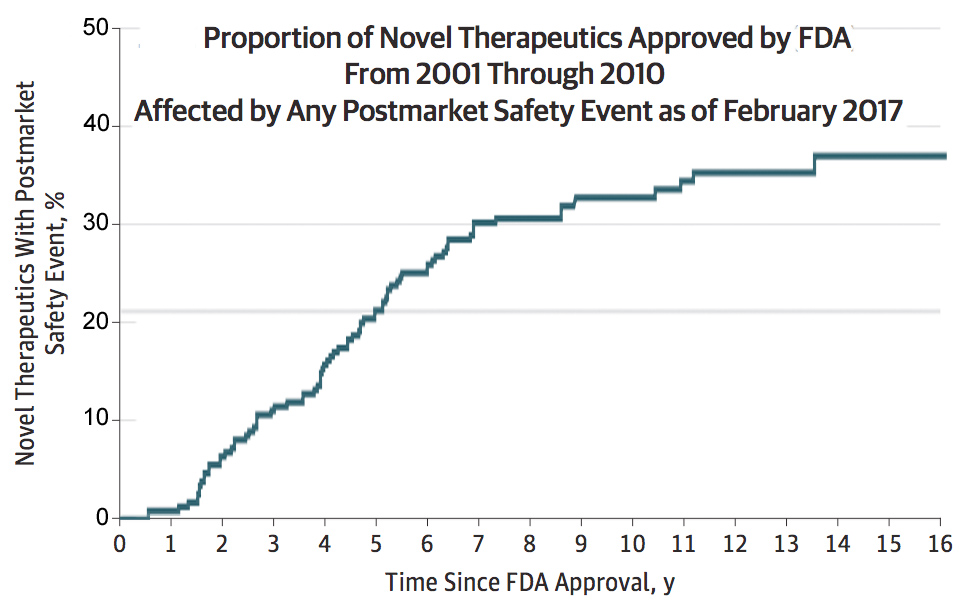
[From jamanetwork.com] Among more than 200 new pharmaceuticals and biologics approved by the U.S. Food and Drug Administration from 2001 through 2010, nearly a third were affected by a postmarket safety event such as issuance of a boxed warning or safety communication, according to a study published by JAMA.
The majority of pivotal trials that form the basis for FDA approval for therapeutics (pharmaceuticals and biologics) enroll fewer than 1,000 patients with follow-up of six months or less, which may make it challenging to identify uncommon or long-term serious safety risks. These risks may only become evident when new therapeutics are used in much larger patient populations and for longer durations in the postmarket period. Postmarket safety events can change how these therapeutics are used in clinical practice and inform patient and clinician decision making.
“The high frequency of postmarket safety events highlights the need for continuous monitoring of the safety of novel therapeutics throughout their life cycle,” the researchers write.
Further Reading:








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




