Pharma Industry News Update: 28 February 2017
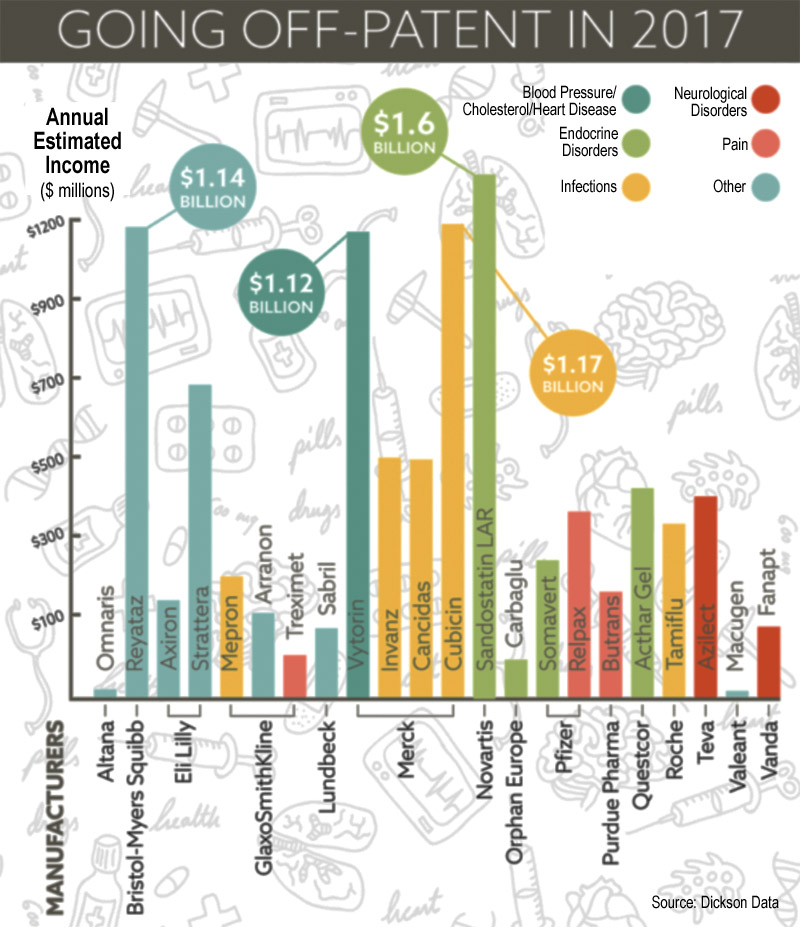
The Top 10 U.S. Drug Patent Losses for 2017
From www.fiercepharma.com
Among the many meds that’ll lose patent protection in the U.S. this year are 10 drugs that each contribute in a big way for top drugmakers. Eli Lilly, Pfizer, Takeda, Bristol-Myers Squibb and Gilead are each set to hit the patent cliff this year with some of their respective big sellers.
Together, they brought in more than $10 billion last year in the U.S. and cover a range of indications: multiple sclerosis, HIV, erectile dysfunction and cancer, among others. They’ll be hot targets as generic rivals rush to steal share with cheaper options.
Further Reading:
- “Lockstep” Price Increases by #Pharma Competitors Keep Rx & Generic Drug Costs High
- Off-patent Drugs at Brand-Name Prices: A Puzzle for Policymakers
![]()
 Will You Miss Those ED DTC TV Ads When Viagra & Cialis Go Generic?
Will You Miss Those ED DTC TV Ads When Viagra & Cialis Go Generic?
From www.forbes.com
[LaMattina, a former Pfizer Executive says…] Once generic competition occurs for a brand name drug, companies generally will stop direct-to-consumer advertising (DTC) for its medication. Why promote a brand name drug in the face of generics? You would just help drive sales of the cheaper generic forms. A company’s DTC budget is better spent on drugs that still have exclusivity.
However, the impact on society for the loss of erectile dysfunction TV ads is unappreciated. No longer will fathers have the educational opportunity to answer the inevitable question by their 10-year-old daughters that arises during Sunday NFL games: “Daddy, what’s erectile dysfunction?”
The subtle reminders of the importance of good hygiene, now promoted by the Cialis commercials with couples in separate bathtubs, will be lost.
[LaMattina, however, suggests that the animated pink intestine that “Crooked Valeant” uses to promote its drug for irritable bowel syndrome will still be around for many years. That’s why “Bubble Guts” is included in Pharmaguy’s “Gallery of Drug Advertising Mascots“.]
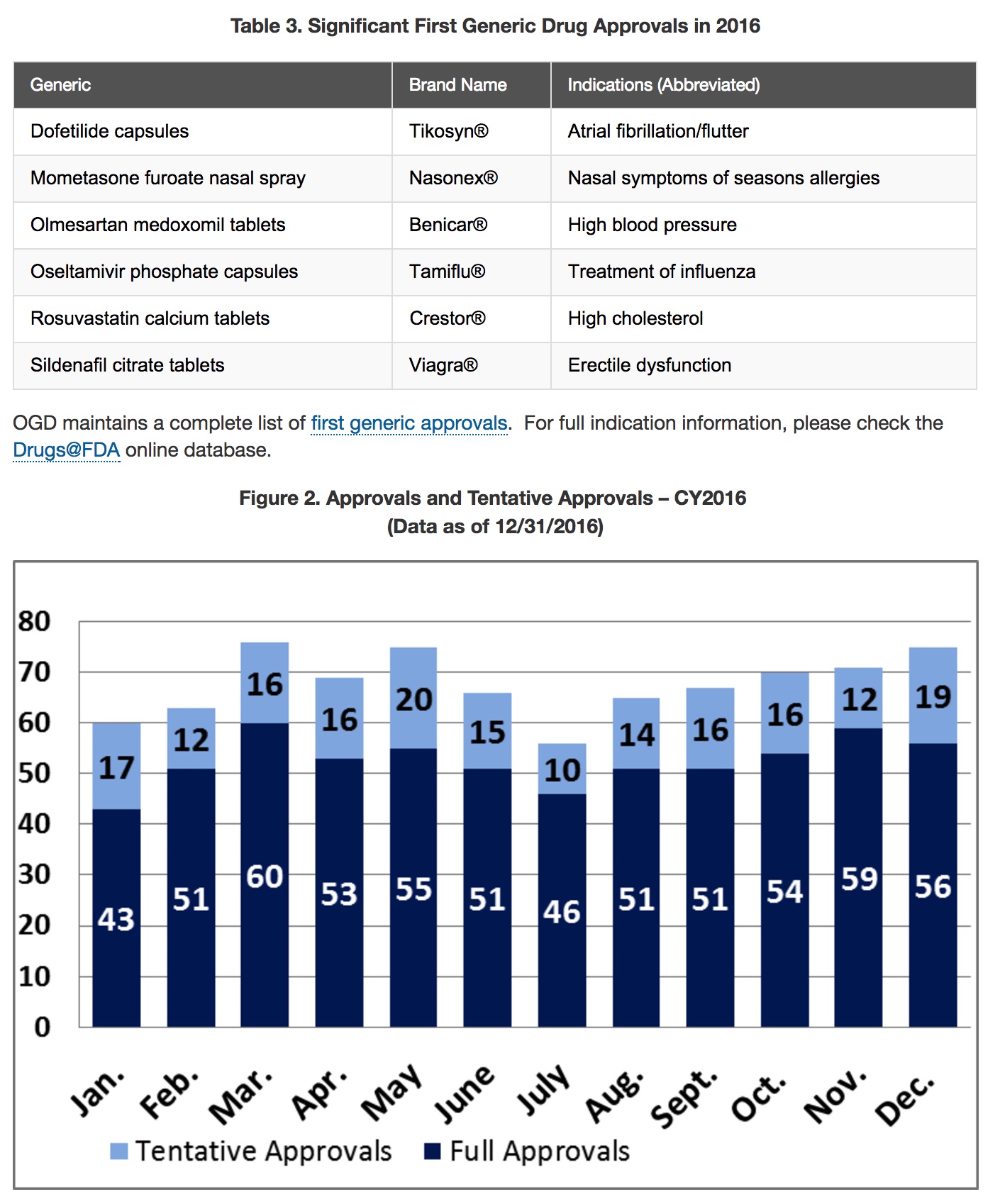
2016: A Record-setting Year for Generic Drug Approvals by FDA
From blogs.fda.gov
Over the last 10 years, generic drugs have saved the U.S. healthcare system about $1.68 trillion. 2016 was a record-setting year for FDA’s generic drug program, a result that will help generate further cost savings for American consumers, while assuring the quality of these generic products.
[FDA approved 630 abbreviated new drug applications (ANDAs) and tentatively approved 183—the highest number of generic drug approvals and tentative approvals in the history of the generic drug program]
“And the timing couldn’t be better amid concerns about rising drug prices,” said FDA. However, read Off-patent Drugs at Brand-Name Prices: A Puzzle for Policymakers.
Further Reading:
- Over 700 Generics Were Approved by FDA in 2015
- FDA Approves First Generic Version of Crestor
- Senators to FDA: “Please Explain” Why the Hell You Stymied EpiPen Competition!









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)