Pharma Industry News Update: 20 January 2017
![]()
 Trump Takes the Wheel to “Make America Great Again!”
Trump Takes the Wheel to “Make America Great Again!”
Today, Donald Trump will be sworn in as America’s 45th President.
It is fitting, therefore, that this issue of Pharma Industry News Update be devoted to Trump’s impact on the pharmaceutical industry.
Articles in this issue of PinUp:
- Pharma Bro’ Says Trump Will “Make Pharma Great Again”
- Novartis CEO Worries About the Market for New Drugs in U.S. After Obamacare is Repealed
- FDA Rushes to Publish Regulatory Guidance Before Trump et al Gut It!
- Trump Names Lawyer Lobbyist Without Any Medical/Science Background At All to Head FDA During Transition
![]()
 Pharma Bro’ Martin Shkreli Says Trump Will “Make Pharma Great Again”
Pharma Bro’ Martin Shkreli Says Trump Will “Make Pharma Great Again”
Martin Shkreli, better known throughout the world as “Pharma Bro,” is betting on Donald Trump to bring pharmaceutical jobs back to the U.S.
“Donald Trump is a dream for business people like me,” Shkreli said during an exclusive interview with FOX Business Network’s Maria Bartiromo. “I think Trump’s going to make pharma great again.”
The former Turing Pharmaceuticals CEO, who first made headlines for sharply increasing the price of a widely-used HIV/AIDS drug (read “Shkreli Surrogates Protest High HIV Drug Prices in London“), said Trump’s proposed policies will make the industry more competitive.
“Almost all pharma manufacturing right now is done in India and China and the quality of manufacturing pharmaceuticals has declined dramatically,” he said. “Bringing those jobs and manufacturing to the U.S. – I think that can only help us.”
More here…
As much as you may hate to admit it, Shkreli has a point: 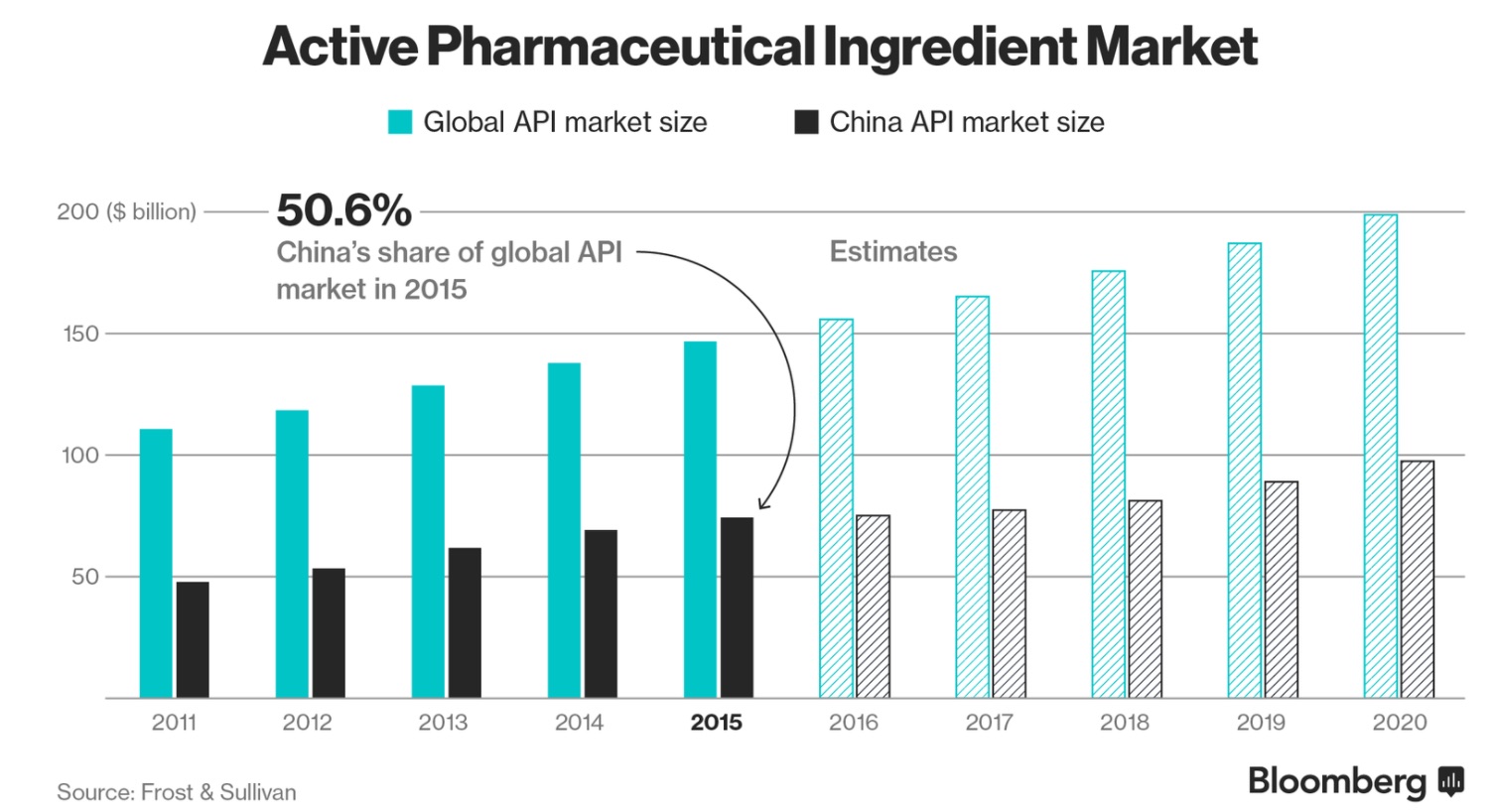
![]()
 Novartis CEO Worries About the Market for New Drugs in U.S. After Obamacare is Repealed
Novartis CEO Worries About the Market for New Drugs in U.S. After Obamacare is Repealed
Donald Trump’s policies could be good for the economy but Obamacare should be replaced if it’s repealed, the CEO of drug giant Novartis told CNBC on Tuesday.
Joseph Jimenez played down some of the negative comments that President-elect has made towards large pharma firms. Trump accused the drug industry earlier this month of “getting away with murder” (read “Trump’s Comments Are Big Pharma’s Nightmare“).
But Jimenez said that some of Trump’s policies could benefit industry.
More here…
Further Reading:
- Donald Trump as President Doesn’t Change the Debate on Drug Pricing, Says Allergan CEO
- Industry Pundits Think Trump is Good for Pharma Advertising & Marketing
- We’re Not Murders, Says Pfizer CEO Ian Read
- Ian Read, Pfizer’s CEO, Not Worried About Trump Lowering Drug Prices
![]()
 FDA Rushes to Publish Regulatory Guidance Before Trump et al Gut It!
FDA Rushes to Publish Regulatory Guidance Before Trump et al Gut It!
President-elect Donald Trump on Friday will become the 45th US president, and though he’s yet to name his choice for commissioner of the US Food and Drug Administration (FDA), the names of those floated to lead the agency and a recent flood of new draft and finalized FDA guidance reveal an agency bracing for change.
[Trump did name someone to head FDA during the transition. Read “Trump Names Lawyer Lobbyist Without Any Medical/Science Background At All to Head FDA During Transition” below.]
Since 7 November, FDA has released almost 20 new draft or revised draft guidance documents, some long-awaited, like the one on biosimilar interchangeability, in addition to more than 20 final guidance documents, a measured explanation of off-label marketing, and an interim policy on drug compounding, as well as a recent launch of a new oncology center.
More here…
Further Reading:
- FDA Throws a “Regulatory Temper Tantrum” Rebuffing Off-Label Drug Promotion Proponents
- Does FDA Guidance About Pharma Communications with Payors Open a Path to Off-Label Discussions?
- Additional Untitled Letters Issued by OPDP Focus on Bottom of Barrel Pharma Companies
![]()
 Trump Names Lawyer Lobbyist Without Any Medical/Science Background At All to Head FDA During Transition
Trump Names Lawyer Lobbyist Without Any Medical/Science Background At All to Head FDA During Transition
Longtime pharmaceutical lobbyist Jack Kalavritinos, who also worked in various capacities for the George W. Bush administration, will take a major role on the “beachhead” team that the incoming Trump administration is sending to run the Food and Drug Administration following Friday’s inauguration, transition team sources say.
Jack Kalavritinos has a law degree from The Catholic University of America, Columbus School of Law. So he’s a religious Catholic and probably opposed to contraception and women’s rights. As far as his pharma experience, he worked for Covidien as a lobbyist (“government affairs advisor”) after various stints working in the Washington bureaucratic “swamp.”
We all know that “interim” FDA heads may hold that position for months!
More here…
Further Reading:








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




