Pharma Industry News Update: 21 December 2015
Featured Free Webinar 
Mastering Mobile Social Media to Improve Health Outcomes
The pharmaceutical industry has made great strides in adopting new technology tools such as social media and mobile apps to communicate with healthcare professionals, consumers, patients, policymakers, and payers. The next frontier is to master these tools in order to improve health outcomes. Access the archived webinar here.
 Gallery of Drug Advertising Mascots Pharmaguy’s Favorite Ad Critters
Gallery of Drug Advertising Mascots Pharmaguy’s Favorite Ad Critters
This is the definitive collection of “mascots” appearing in direct-to-consumer (DTC) drug ads going back to the dawn of the genre.
The slide deck includes such favorites as:
- Enablex Water Balloons
- The Myrbetriq Bladder Boy (or is it a girl?)
- The Petrol Lady (now retired)
- Vesicare Pipe People
- The Xifaxan Gutsy Bubble Boy
- Rapaflo’s “Wally” the Walnut, er, Prostate
- Lamisil’s Digger the Dermatophyte
- and many more…
 What the Drug Industry Can Learn from FDA’s Social Media Policy Reading Between the Lines
What the Drug Industry Can Learn from FDA’s Social Media Policy Reading Between the Lines
The FDA recently posted a new social media policy, but it’s not for the pharmaceutical industry. The policy is for its own employees.
Like similar policies developed by pharma companies such as Roche, FDA’s SM policy provides guidance to their employees about how to use social media for personal and professional purposes.
“The FDA encourages the use of social media technologies to enhance communication, collaboration, and information exchange in support of FDA’s mission to protect and promote public health,” states the introduction. “This policy applies to FDA employees, contractors, and other personnel acting in an official capacity when using social media to communicate with the public regarding FDA-related matters.”
Reading “between the lines” of this policy offers some hints as to how the FDA may further regulate pharma’s use of social media in the future, especially with regard to correcting/deleting comments posted to its own sites and linking to external third-party sites..
Read more here.
Apple Watch Was Dumbed Down To Avoid FDA’s Blurred Regulatory Lines 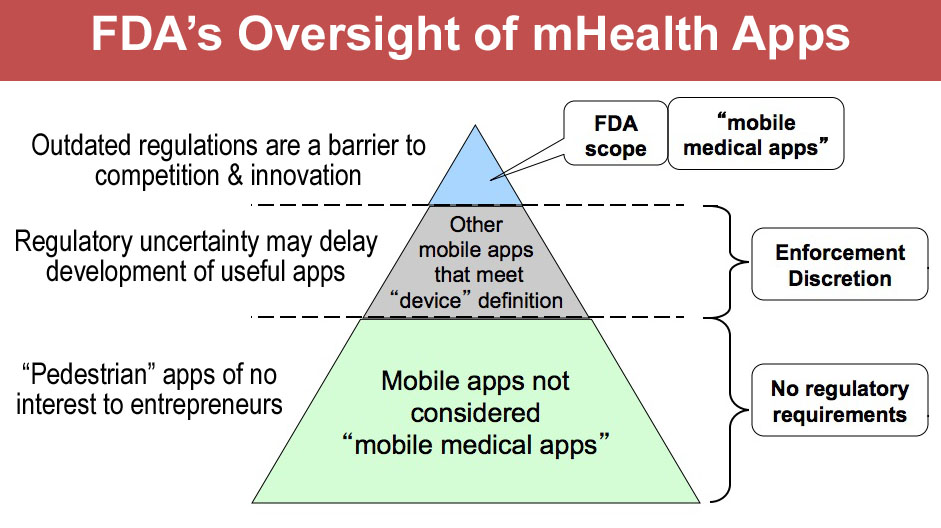
As advances the medical promise of its watch and smartphones, it has also made clear that its foremost aim is to steer clear of Food and Drug Administration regulation. Many assert that this imperative has rendered the health features in its sensor-laden watch underwhelming. Apple didn’t want to cross the lines that would class its new watch as a “medical device” in the eyes of regulators.
Those lines are increasingly murky. FDA put out guidance earlier this year meant to set a boundary between digital tech not subject to its oversight, and the products that regulators want to review. FDA billed the document as an act of “de-regulation” because agency staff asserted whole categories of products that they didn’t seek to actively supervise. But the stuff FDA excluded was pedestrian. The scheme they reserved for the vast rest of the digital health tools wasn’t well articulated.
Read more here.
 Pharma Should Not Be Afraid to Develop Digital Apps For Physicians & to Improve Outcomes
Pharma Should Not Be Afraid to Develop Digital Apps For Physicians & to Improve Outcomes
When it comes to the pharmaceutical industry’s digital ambitions, it’s becoming less and less about producing stand-alone apps and more about developing digital tools that have therapeutic potential. This was the message Dr. Joseph Kvedar had for those attending the recently concluded 2015 HIMSS Connected Health Conference.
According to Kvedar, executives at pharmaceutical companies generally have viewed digital technologies from a marketing perspective. But, pharma’s marketing-focused approach toward digital health is beginning to change. One major factor contributing to pharma’s evolving relationship with digital is an ongoing effort to figure out how to deliver products and services that go “beyond the pill,” and improve well-being while boosting outcomes.
According to MobiHealth News, Kvedar noted, that “we’re seeing more instances where [pharmaceutical executives] are adding a wearable component as part of the package or an app that is an integral part of the therapeutic.” He urged more drug firms to “start thinking quite differently about the space, and not everything that makes you better is a pill.”
More on this here.
Not such a long time ago in a pharma company pretty close by… 
Featured Survey 
Use of Behavioral Targeting by Pharma Marketers This survey solicits your opinion on the appropriateness of using behavioral targeting techniques in online pharma marketing. After taking the survey you will have access to a summary of results to date.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




