Pharma Industry News Update: 7 December 2015
Featured Survey 
Use of Behavioral Targeting by Pharma Marketers This survey solicits your opinion on the appropriateness of using behavioral targeting techniques in online pharma marketing. After taking the survey you will have access to a summary of results to date.
 FDA Releases Social Media Policy Provides Scientists Freedom of Tweet Speech
FDA Releases Social Media Policy Provides Scientists Freedom of Tweet Speech
The FDA just unveiled its new social media policy, which provides guidance to their employees on use of social media for official and personal purposes.
The policy earns an A, or 90 out of 100 points on the scale created for the Union of Concerned Scientists’ Grading Government Transparency report. This puts the FDA near the top of the class in terms of policy quality. By comparison, other agencies ranged from a D to an A on this scale. Here’s what earned the FDA high marks.
A substantial portion of points came from its distinction between official and personal use of social media, and notably, the freedom it gives to its employees on the latter. Not all agencies’ policies made this important distinction in clarifying how their employees can use social media and only a few match or exceed the FDA policy’s excellent guidance on personal use of social media.
The FDA policy states, “To use social media in his or her personal capacity, an employee does not need to obtain permission or approval…” and “an employee may include his or her title of position in an area of the social media account designated for biographical information.” The policy also encourages use of a disclaimer if employees have concerns that use of social media may create the impression of agency views. Other agencies with strong language in this area are the Department of the Interior and the National Institutes Health social media policies FDA provides their scientists with the right to corrections on social media.
How does this policy compare with pharma industry policies?
More details here.
 Pharma and mHealth Device Maker Partnerships E.g., GSK & Propeller Health
Pharma and mHealth Device Maker Partnerships E.g., GSK & Propeller Health
The shift to value-based care is creating partnerships between mobile device makers and Big Pharma.
Propeller Health, for example, provider of an FDA-cleared digital health solution for improving outcomes in asthma and chronic obstructive pulmonary disease (COPD), today announced a development agreement and R&D collaboration with GSK for its Ellipta® inhaler, the pharmaceutical company’s innovative, patented, dry powder inhaler.
Under the terms of this non-exclusive agreement, the first between the two companies, Propeller Health will develop and manufacture a custom sensor for GSK’s clinical studies with the Ellipta inhaler in a number of respiratory diseases.
In the studies, the sensor will automatically collect and record data on the inhaler’s usage (such as date and time of each use), wirelessly transmitting the information to a central data repository for analysis by GSK’s clinical researchers. The sensor technology will be used to provide greater insights into adherence patterns across patient populations and will allow for more precise correlation of adherence with safety, efficacy and economic outcomes.
Read more here.
Street Prices of Rx Drugs Rise Dramatically Study of retail prices 19 brand-name prescription dermatologic drugs 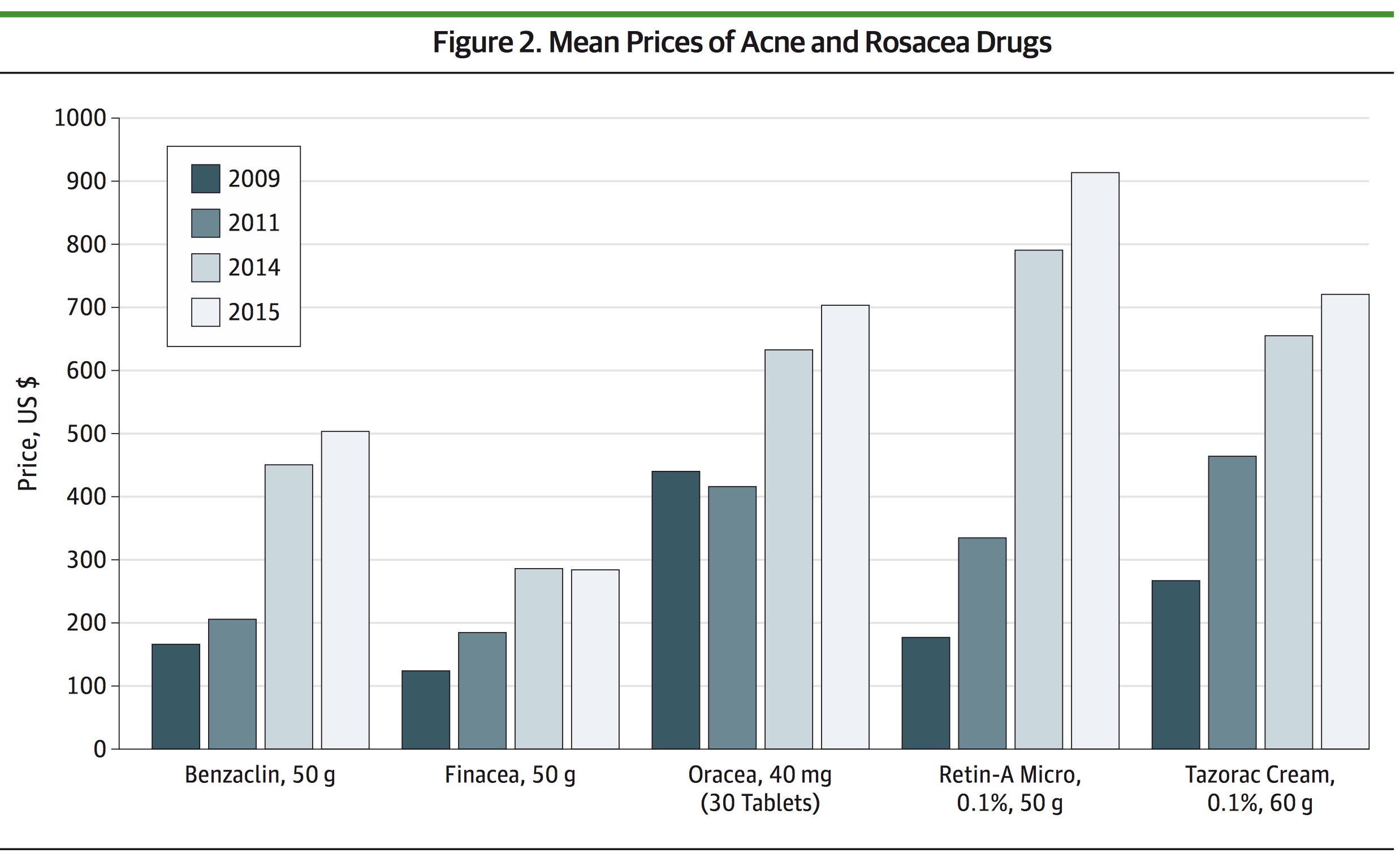
Drug companies often claim that the wholesale price of drugs is not an good indicator of what patients, insurers, and the government pay (see here, for example). So a new study published in JAMA Dermatology looked at the retail prices 19 brand-name prescription dermatologic drugs sold at four national chain pharmacies in the West Palm Beach, Fla., area (Costco, CVS, Sam’s Club and Walgreens) in 2009, 2011, 2014 and 2015. The authors found that between 2009 and 2015 prices of all surveyed classes of brand-name drugs increased; the average increase was 401 percent. Prices of topical antineoplastic drugs had the greatest average absolute and percentage increase of nearly $10,927 and 1,240 percent. “Percent increases for multiple, frequently prescribed medications greatly outpaced inflation, national health expenditure growth, and increases in reimbursement for physician services,” the study concludes. There was one surprising finding.
Read more here.
 Patients with Chronic Diseases Want Better Mobile Apps To Bring Digital Info to Docs, Not Vice Versa
Patients with Chronic Diseases Want Better Mobile Apps To Bring Digital Info to Docs, Not Vice Versa
A new study released by mobile engagement provider Mobiquity exposes the “gap between patients’ demand for taking control of their own health and the accessibility or availability of digital and mobile tools when it comes to the management of chronic health conditions.”
The study revealed that one third of patients with chronic diseases don’t currently use mobile apps to manage their conditions, but would like to start.
In fact, the report summary notes, one in four respondents feel that “wearable devices are the way of the future.”
Interestingly, almost 50 percent of patients believe they should bring information/digital tools to their doctor – rather than the other way around – reinforcing their desire to be actively involved in managing their health rather than trust their doctors to exclusively manage it.
When asked about the most challenging aspects of managing their conditions, 26 percent of respondents agree that finding direct means of communicating with health professionals presents the biggest hurdle.
More on this here.
Cartoon: Docs on Pharma Payroll 









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




