Pharma Industry News Update: 19 July 2016
![]()
 GSK Develops Rheumatoid Arthritis App for Clinical Trial Using Apple’s ResearchKit
GSK Develops Rheumatoid Arthritis App for Clinical Trial Using Apple’s ResearchKit
GlaxoSmithKline Plc has started a rheumatoid arthritis study using Apple Inc.’s ResearchKit, marking the first time a drugmaker has used the health system for the iPhone to conduct clinical research. Glaxo wants to record the mobility of 300 participants over three months and will also ask the patients to input both physical and emotional symptoms, such as pain and mood.
The success of the study could help determine the pharmaceutical industry’s future appetite for using Apple’s products to conduct research.
More here…
![]()
 Too Many mHealth Apps Are Failing the Usability Test
Too Many mHealth Apps Are Failing the Usability Test
A new UCSF study finds that some of the most popular apps for diabetes, depression and caregivers are too difficult to use, especially by underserved populations.
In many cases, patients had difficulty accessing features or entering data, and in almost every case the patient didn’t get far enough to use that data. Over the course of the study, the patients only completed about half of the data-entry tasks, and only 43 percent were able to retrieve data from the apps.
This is not good news for pharma companies that wish to use mHealth apps to collect data from clinical trial participants. GSK, for example Developed a Rheumatoid Arthritis App for Clinical Trial Using Apple’s ResearchKit (see here). Even using this type of advanced technology does not guarantee that the app will be used to its full extent. It will be interesting to see if GSK reports on the usability of its app in the real world of clinical trials.
More here…
![]()
 Poof! FB & SnapChat Offer #pharma Marketers Transient, Untraceable Messaging
Poof! FB & SnapChat Offer #pharma Marketers Transient, Untraceable Messaging
Taking a page from Snapchat, Facebook this week began testing disappearing messages for its Messenger service. But pharma marketers may want to hold off on embracing the new tool.
One of the reasons for the popularity of time-restricted messaging such as SnapChat and now Facebook messaging, is it taps into Pharma’s FOFDAR (fear of FDA regulations). Because the content disappears in 24 hours, the chances of the FDA seeing it and documenting violations is minimized. Of course, pharma companies must submit promotional material to FDA at the time of distribution, but by then the promotion is gone.
More here…
![]()
 PhRMA Welcomes Five New Member Companies, Including Teva
PhRMA Welcomes Five New Member Companies, Including Teva
“On behalf of the PhRMA Board of Directors, I am proud to welcome these companies into our membership,” PhRMA chairman and Biogen CEO George A. Scangos said. “Together as an industry, we can continue our work on issues to advance biomedical innovation and improve patient care.”
At first, it was uncertain if PhRMA would accept Teva, a generic drug company, into its ranks. For more on that, read “Teva to PhRMA: ‘Can’t We All Just Get Along?’“
More here…
Which #Pharma Companies Have the Most Branded Drugs?
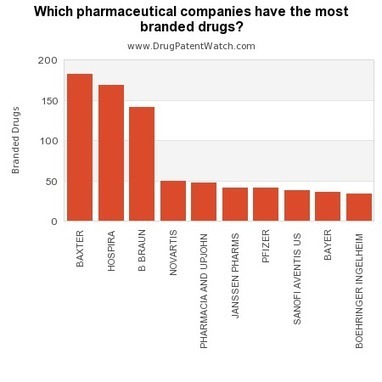
This chart shows the pharmaceutical companies with the most branded drugs. But there’s something strange about it.
Learn what’s strange here…








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




