Pharma Industry News Update: 5 April 2016
 Culturally-Relevant Health Marketing: Boehringer Engages U.S. Hispanics About Diabetes
Culturally-Relevant Health Marketing: Boehringer Engages U.S. Hispanics About Diabetes
Pharmaguy interviews Lisa Valtierra, former Associate Director of Cross Cultural Marketing at Boehringer Ingelheim. We discuss how Boehringer Ingelheim engaged U.S. hispanics about diabetes in a culturally-relevant manner via TV, mobile, and the Web.
More info here, including link to podcast.
 History Lesson: How the “Girl from Google” Got Pharma Into Trouble with the FDA
History Lesson: How the “Girl from Google” Got Pharma Into Trouble with the FDA
“I could strangle that girl from Google,” said a friend of mine after a presentation by a Google operations specialist at a 2006 eyeforpharma eCommunications and Online Marketing conference held in Philadelphia. Of course, this was not a literal terroristic threat, just an expression of frustration by a long-time industry advocate.
The “Girl from Google” (GfG) was up just before my presentation and the title of her talk was “The Importance of Interactivity: How multimedia technologies will change the way you Connect with Consumers & Physicians.”
What did the “Girl from Google” (GfG) say that upset this person so much?
Find out here.
Thanks to Cartoon Characters, We Know How Belsomra Works, But It Doesn’t Work Well  After Belsomra hit the market, Consumer Reports asked Dr. Lisa Schwartz, a professor at Dartmouth Medical College, to create a label for it. Her version presents the data on the drug in an even-handed way, noting that its ability to aid sleep is “modest” at the highest approved doses. “Short track record means that new, unexpected side effects are possible,” it explains. “Since this drug has a different way of acting than other insomnia drugs, the experience with it is particularly limited.” The label gives brief details on alternative remedies for insomnia, like cutting down on caffeine. Finally, it lists Belsomra’s known side effects. Not included on the list but probably warranted: skepticism.
After Belsomra hit the market, Consumer Reports asked Dr. Lisa Schwartz, a professor at Dartmouth Medical College, to create a label for it. Her version presents the data on the drug in an even-handed way, noting that its ability to aid sleep is “modest” at the highest approved doses. “Short track record means that new, unexpected side effects are possible,” it explains. “Since this drug has a different way of acting than other insomnia drugs, the experience with it is particularly limited.” The label gives brief details on alternative remedies for insomnia, like cutting down on caffeine. Finally, it lists Belsomra’s known side effects. Not included on the list but probably warranted: skepticism.
More about this here.
 2nd FDA “Warning Letter” of 2016 Cites Patient Assistance Voucher
2nd FDA “Warning Letter” of 2016 Cites Patient Assistance Voucher
The US Food and Drug Administration’s (FDA) Office of Prescription Drug Promotion (OPDP) has found that Shionogi’s “Patient Co-Pay Assistance Voucher” for Ulesfia (benzyl alcohol) lotion to help treat head lice is false or misleading because it omits important risk information.
This is OPDP’s second untitled letter of 2016 (the first was for a Pfizer/Hospira YouTube video), and the office makes clear that promotional materials “are misleading if they fail to reveal facts that are material in light of the representations made or with respect to consequences that may result from the use of the drug as recommended or suggested in the materials.”
In the case of the voucher, it makes representations and/or suggestions about the efficacy of Ulesfia such as that it’s “the #1 prescribed branded Rx treatment for head lice” and also that it’s made up of “benzyl alcohol 5%, this is a non-neurotoxic formulation, and is not a pesticide and is the #1 prescribed branded Rx product for the treatment of head lice.”
More here.
Public Citizen: Regulators Must Be Tougher Against Pharma Fraud 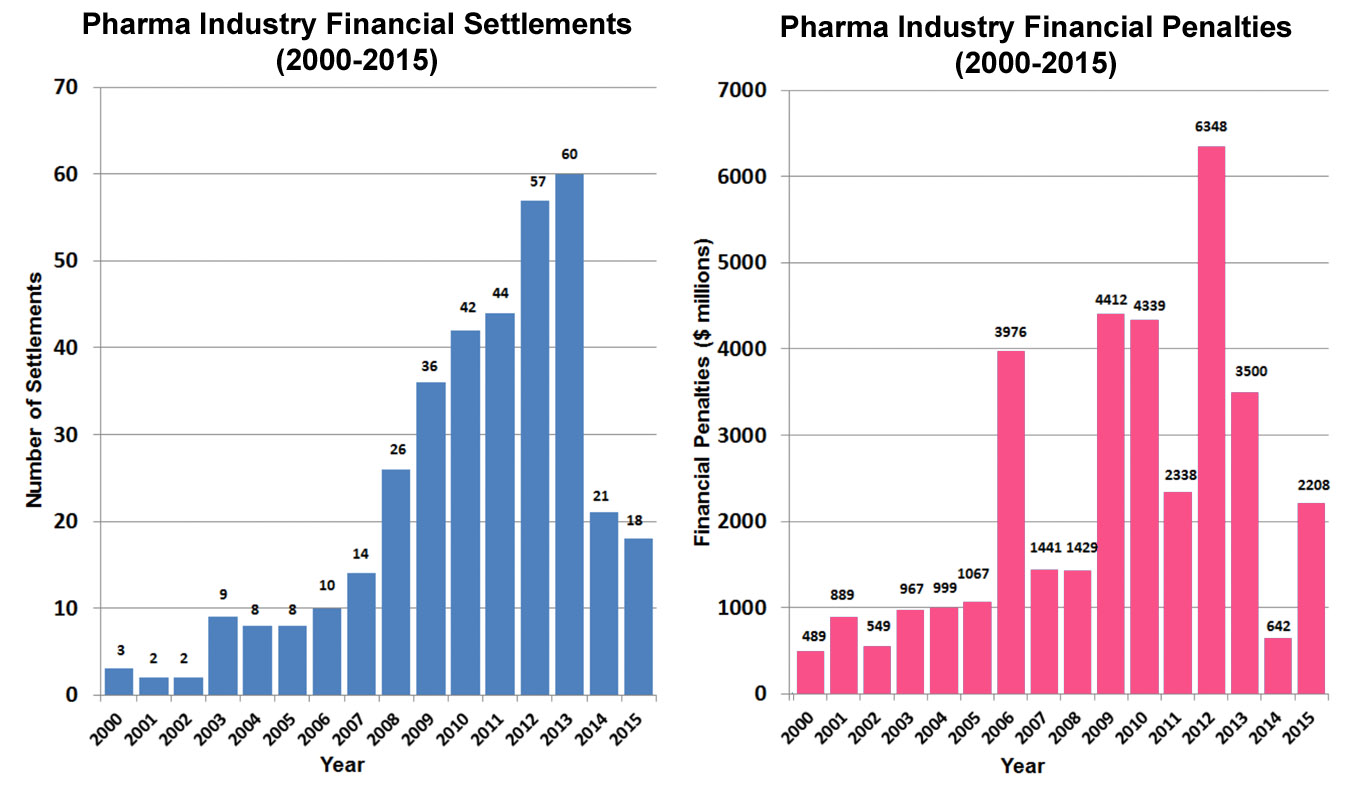 Read the details and examine the evidence here.
Read the details and examine the evidence here.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




