Pharma Industry News Update: 26 January 2016
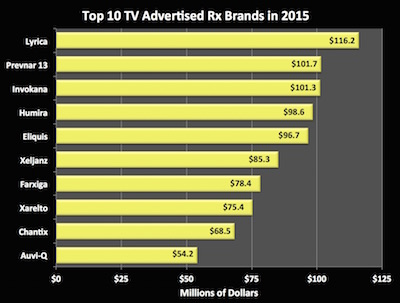 Top 10 Rx TV Ad Spends Pfizer Continues to Dominate!
Top 10 Rx TV Ad Spends Pfizer Continues to Dominate!
Pfizer owned TV ad spending for 2015 among pharma brands. It took 5 spots on the top 10 list for the year, including Nos. 1 and 2, according to iSpot.tv estimated spending tallied for FiercePharmaMarketing.
Seizure and pain drug Lyrica came in first with $116.2 million spent in 2015, while Pfizer’s pneumococcal pneumonia vaccine Prevnar13 came in at No. 2 with $101.7 million.
The three other Pfizer drugs on the list were anticoagulant Eliquis (No. 5), arthritis fighter Xeljanz (No. 6) and smoking cessation drug Chantix (No. 9), spending $96.7 million, $85.3 million, and $68.5 million respectively, according to iSpot.tv data.
More here.
 FDA’s 1st 2016 Enforcement Letter Goes to… Pfizer!
FDA’s 1st 2016 Enforcement Letter Goes to… Pfizer!
In the first enforcement action from the FDA’s marketing police this year, the Office of Prescription Drug Promotion put Hospira in the hot seat over a YouTube video for its sedative Precedex.
The OPDP sent an untitled letter dated Jan. 14 to the Pfizer-owned company, charging the video “omits risks and material facts” about the drug. The agency also rebuked Hospira for publishing the promotional video without submitting it to the OPDP for review.
Although Pfizer said the video cited by FDA was removed from Youtube et al, there is another copy still on YouTube here. This was uploaded a few years ago by Ayman Farouk, former Marketing Manager Hospira EGYPT. Find his LinkedIn profile here.
More here.
FDA Expedites Drug Approvals, But… Its Postapproval Oversight Stinks
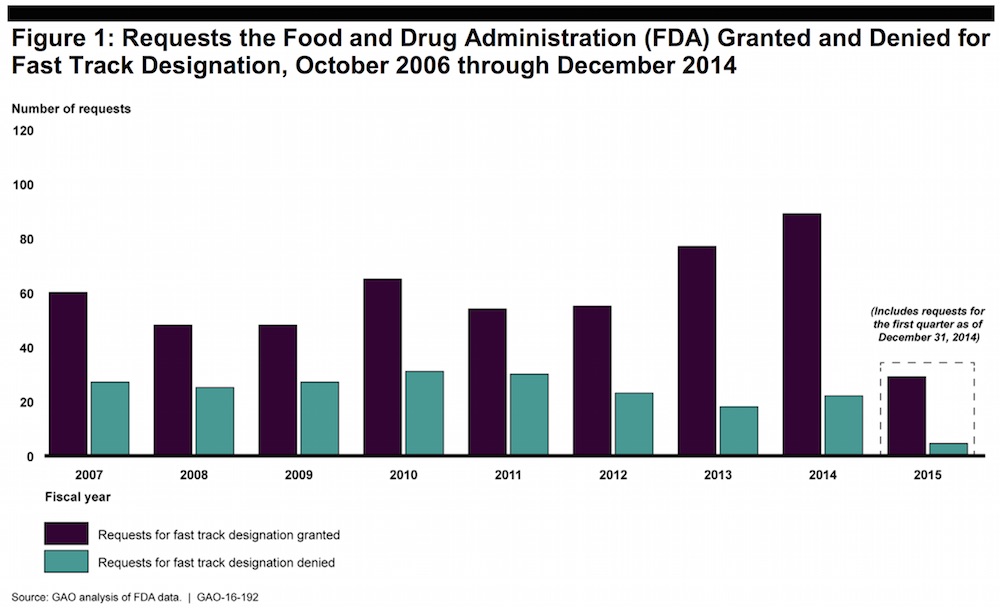 “FDA has supported efforts to shorten development and streamline the agency’s review of drug applications through expedited pathways,” says the GAO. “However, we found problems with the agency’s efforts to oversee and track potential safety issues and postmarket studies once those drugs are on the market.”
“FDA has supported efforts to shorten development and streamline the agency’s review of drug applications through expedited pathways,” says the GAO. “However, we found problems with the agency’s efforts to oversee and track potential safety issues and postmarket studies once those drugs are on the market.”
“FDA lacks reliable, readily accessible data on tracked safety issues and postmarket studies needed to meet certain postmarket safety reporting responsibilities and to conduct systematic oversight,” says the GAO.
More here.
 Social Media Listening and “Patient Centricity” What’s the Right Goal?
Social Media Listening and “Patient Centricity” What’s the Right Goal?
According to Richie Etwaru, chief digital officer at IMS Health, “by listening to customers, and working to understand the good, bad and ugly parts of the patient experience, life-sciences companies can become patient-centric and move from “dislike” to “like.” Only then will they buy more of our products and services.” “This underscores why pharma may be taking the wrong approach to patient centricity, IMO,” said Pharmaguy. “If you are into patient-centricity to sell more products, you’re going to be moving in the wrong direction and end up disappointed.” So, in how is pharma more successful being patient-centric?
More about that here.
Cartoon: New Age Marketing!

Featured Survey Tell Us About Your Social Media Listening Program
 Social media listening is a hot topic these days. There is no doubt that collecting information from disease and product conversations on social media sites helps pharmaceutical companies develop more impactful messaging and marketing tactics.
Social media listening is a hot topic these days. There is no doubt that collecting information from disease and product conversations on social media sites helps pharmaceutical companies develop more impactful messaging and marketing tactics.
This survey is an attempt to understand how pharmaceutical companies and their agencies are using social media listening programs as a part of their marketing and communications programs.
CLICK HERE to take the survey.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




