I just received a link to an old Powerpoint (PPT) presentation that was featured at an October, 2002, Pharmaceutical Marketing Research Group (PMRG) conference. The presentation was entitled “Positioning Detrol (Creating a Disease)” and the presenter was Neil Wolf who, at the time, was Group Vice President at Pharmacia, the drug company purchased by Pfizer. As you may know, Pfizer is preparing Toviaz, a follow-on drug to Detrol, for marketing (see “Detrol v. Toviaz: Marketing Replaces Innovation at Pfizer!“).
The presentation outlines the strategies used to convert a “niche product into a Mass Marketing Opportunity.” Download it here.
I was gratified to get my hands on this PPT because I remembered being present at the 2002 meeting and how shocked I was that a pharmaceutical VP would actually be so transparent in discussing how “overactive bladder” was a “new” disease that he and his company created specifically to increase the sales of Detrol! Often, I wished I had a copy of that presentation to prove that I wasn’t imagining things and now here it is.
The introductory notes state:
How many have seen
How many have heard of overactive bladder?
How many have overactive bladder???
Overactive Bladder now a lexicon in American culture
-cocktail parties / media / sitcoms / talk shows
Most importantly, routinely discussed between patients and their physicians
Not the case 4 years ago
Pharmacia instrumental in creating new disease
-improving lives of millions around world
Some critics of the pharmaceutical industry say that overactive bladder (OAB) is an example of “disease mongering,” a term that was coined by the late journalist Lynn Payer to describe what she saw as the confluence of interests by some doctors, drug companies, patient advocacy groups and media in exaggerating the severity of illness and the ability of drugs to “cure” them (see “Disease Mongering — Is it real or not?“).
I believe this presentation is a classic “disease mongering” case study.
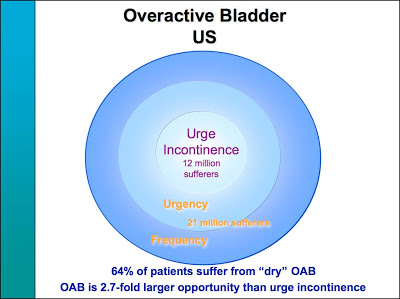
Slide #20 shows how by creating a “new” disease, the potential market for Detrol could be increased 2.7-fold!
Slide #22 states the “Critical Success Factors” for the Detrol launch in the US:
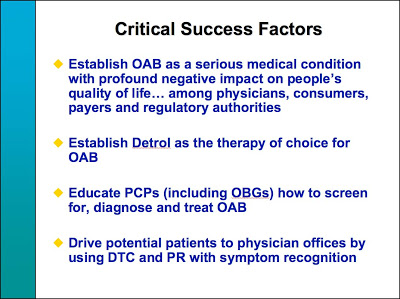 This is now the classic formula for creating greater demand for an Rx product than would normally be the case. As you can see, “education” of PCPs (primary care physicians) is a critical component as is DTC and PR (public relations).
This is now the classic formula for creating greater demand for an Rx product than would normally be the case. As you can see, “education” of PCPs (primary care physicians) is a critical component as is DTC and PR (public relations).
NOTE: Influencing Prescribers
The American Medical Women’s Association (AMWA), which is cited as a resource on the Detrol product Website, is currently promoting an “Overactive Bladder Initiative,” which includes a “comprehensive continuing medical education curriculum” that provides “primary care providers with the latest discoveries concerning etiology, epidemiology and treatment of urinary incontinence, with particular emphasis on practical management strategies of overactive bladder, particularly as it affects women.” AMWA lists Abbott, Novartis, Astrazeneca, and Wyeth (recently purchased by Pfizer) as corporate sponsors.
Slide #30 in the deck clearly shows that Pharmacia understood the need to achieve the “confluence of interests by some doctors, drug companies, patient advocacy groups and media” as well as REGULATORY people to achieve its aims:
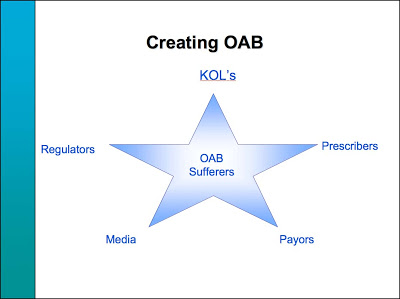
To get payors (ie, insurance companies) to place Detrol on their formularies and pay for OAB treatments, Pharmacia convinced them that OAB was “a serious medical condition, not just a ‘lifestyle’ disorder” citing “Skin and soft tissue infection,” “Falls and fractures,” “UTIs,” and “Significant co-morbidity with depression and sleep disorders” (slide #39).
Interestingly, none of these “serious” conditions was ever mentioned in the DTC marketing of Detrol. That might be just too much education for consumers to swallow!
Continue the discussion via Twitter…
————–
Listen to this WHYY radio segment, which aired Monday, April 20th, 2009:








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




