At the recent FDA public hearing, Google presented its “ideas” for standard paid search ads for Rx products. The following, for example, shows its “proposal” for “Black Box Sponsored Links:”
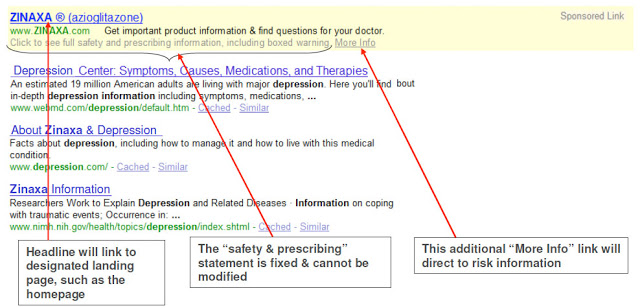 |
| Click on image for enlarged view. |
According to Klick Pharma’s “Applying FDA Regulations to Online Marketing” guide, “Products with boxed warnings do not have the same flexibility in terms of creating reminder ads, as this form of ad is not permitted by the FDA for such drugs. While many boxed warning drugs have and continue to use branded reminder ads for search, it is not advisable given the current environment.”
Bayer has chosen not to heed this warning and decided to use Google’s new “proposed” format for its YAZ search campaign, as shown in this screen shot below (the ad I am referring to is the one on top, :-):
YAZ is a special case. Not too long ago, a YAZ TV ad was cited by the FDA for violating its regulations (see “FDA and YAZ: Is FDA Helping Marketers Work Around Regulations?“). My criticism may have helped goose the FDA to do something it rarely does: require Bayer to run new ads to correct previous YAZ marketing. Also, Bayer entered into a settlement with several states in which it agreed to submit all YAZ ads for federal screening before they appear. The term of the agreement was 6 years.
Does this agreement include paid search ads such as the one above, or does it pertain only to TV ads? Did the FDA “screen” this YAZ sponsored Google ad? What does “screening” by the FDA mean?
Or, is the FDA irrelevant? Is Google the new FDA?
With apologies to Bob Dylan:
You’ve been with the Google geeks
And they’ve all liked your looks
With great lawyers you have
Discussed black boxes and crooks
You’ve been through all of
Josh Bernoff’s books
You’re very well read
It’s well known
But something is happening here
And you don’t know what it is
Do you, Mister FDA?









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




