The pharmaceutical industry is often criticized for developing new Rx drugs having little value over current medications. Such drugs are called “me-too” drugs because of their similarity to existing Rx drugs. Glaxo has now taken this to a completely NEW level: developing an Rx drug that is a “me-too” of existing over-the-counter dietary supplements. I am talking about LOVAZA, aka omega-3-acid esters approved by the FDA for the treatment of high triglycerides. Here’s the ad for LOVAZA, which I cut out of this week’s Newsweek Magazine (click on it for an enlarged view):
LOVAZA seems identical to the Member’s Mark brand of Omega 3 Fish Oil that I can buy at my SAM’s Club store for about $18 a bottle. This bottle includes 400 Softgels and will last me 3-4 months if I take the recommended 4 1000mg softgels per day — that works out to be about $5-6 per month.
Is LOVAZA different than the OTC (over-the-counter) product I have in my kitchen cabinet? Here’s how they compare (check out the LOVAZA package insert here):
Dosage Form: MM, 1000 mg softgel; LOVAZA, 1000 mg softgel
Dosage: MM, 4 softgels per day; LOVAZA, 4 softgels per da
Active Ingredients: MM, 300 mg of 3 Fatty Acids (EPA and DHA); LOVAZA: Each 1-gram capsule of LOVAZA contains at least 900 mg of the ethyl esters of omega-3 fatty acids sourced from fish oils. These are predominantly a combination of ethyl esters of eicosapentaenoic acid (EPA – approximately 465 mg) and docosahexaenoic acid (DHA – approximately 375 mg).
Hmmm… the major difference is that LOVAZA contains more EPA and DHA than does the Member’s Mark OTC version (465 mg of EPA and 375 mg of DHA vs 300 mg of EPA+DHA, respectively). That’s about 2.8X more, which means I would have to take 11 MM softgels per day to equal 4 LOVAZA sofgels (the LOVAZA Web site says “It could take up to 14 capsules per day of an omega-3 supplement to provide the same amount of active ingredients proven to lower very high triglycerides”). My 400 MM softgels would then last me only 1 month or so. That raises my monthly cost to about $18. Bummer!
But wait! What if I were prescribed LOVAZA? How much would it cost me? I’m willing to bet that my insurance company might balk and make the co-pay very high — say $25 — or insist on substituting an OTC equivalent, even it means I have to take more pills per day.
So, LOVAZA is likely to cost me about 38% more than Member’s Mark Omega-3, but be more convenient because I only have to take 4 of them per day. Note: GSK offers a savings card that allows me to save up to $20† off each of my next 12 refills of LOVAZA. I’ve tried to find the footnote referenced by that (†) symbol, but can’t seem to find it on the Web site. My old eyes may not be good enough. Besides, I probably don’t qualify for the full $20 discount — I never win anything!
My prediction is that LOVAZA is not going to be popular with people like me — older men with high triglycerides. First, I hate refilling prescriptions and if you think I am going to carry around ANOTHER card in my wallet, you’re crazy! Secondly, I am already pissed off that as soon as Abbott came out with its industrial-strength Rx version of NIACIN in combination with something else (I forget exactly what), I can no longer find the right kind of OTC niacin in CVS as I used to. I suspect that CVS took it off the shelf in order to sell Abbott’s Rx version (previously, the CVS pharmacist recommended the OTC version, now he is silent about it).
I would love to have been part of the focus group that GSK studied before it began marketing LOVAZA. You did have a focus group, didn’t you?
————-
Afterthought:
Why Did the FDA Approve LOVAZA In the First Place?
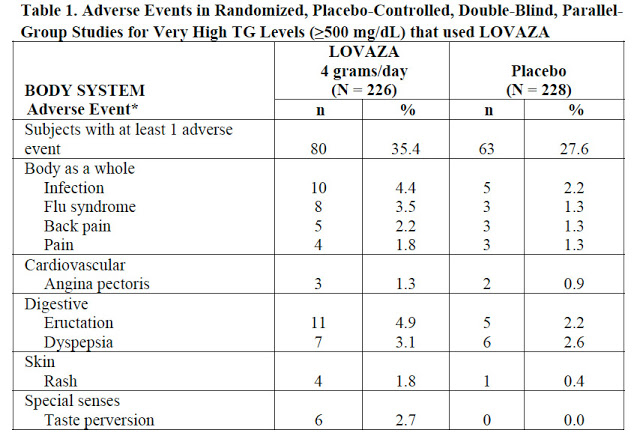
Nevermind that this is a “me-too” product that has no business being an Rx drug because there are so many OTC equivalents out there. It is also of dubious value because the data supporting its effectiveness (vs. placebo) is questionable. Here’s a table from the prescribing information relating to adverse events:
Note the N values: 226 and 228! If these are the number of patients in the clinical trial, as I suspect they are, then the trial did not include enough patients to prove anything, let alone effectiveness! I am certain you cannot get from 228 data points any significant results that rise above the level of pure chance!










![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




