Many pharmaceutical corporate Twitter accounts are devoted to posting news about the company, including news about new research results relating to one of its products. In these tweets, product names are mentioned as well as the research results, but no fair balance information is included. Such tweets include links to press releases, which include all the necessary risk information.
I am seeing a lot of these tweets and wonder if they violate FDA regulations.
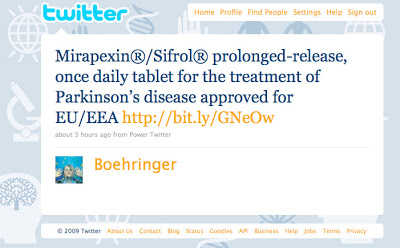
Back in October, 2009, I criticized such a tweet by Boehringer Ingelheim (BI), which I thought did violate FDA regulations (see “Boehringer’s Branded Tweet Violates FDA Regulations Just Like Those 14 Paid Search Ads Did“). The tweet is shown below:
In it’s comments to the FDA, PhRMA, the US drug industry trade association, included a section about “Responsible Microblogging of Newsworthy Events.” PhRMA stated that the “FDA has set a responsible example in its use of Twitter to broadcast newsworthy events such as new drug approvals. Given the limited space constraints (e.g., 140 characters) of such media, and consistent with FDA’s own use of such media [emphasis added], the Agency should allow biopharmaceutical manufacturers to microblog about significant scientific and regulatory events (e.g., approvals, new indications, recalls) for a medicine, provided that (i) all information provided in the initial entry is truthful and accurate, and (ii) the landing page contains a comprehensive description of product risks and benefits. As FDA’s regulations recognize, scientific exchange of information which is neither advertising nor promotion can and should exist, and manufacturers should serve as responsible stewards of such information about the products they develop.”
PhRMA cited this example of a FDA tweet announcing the approval of a new product: “FDA Approves Votrient (pazopnib), a New Treatment for Advanced Form of Kidney Cancer. http://bit.ly/Votrient” A more recent example from the FDA Twitter people is shown below:
I must say I agree with PhRMA’s logic: if it’s OK for the FDA to do it, then it should also be OK for pharma companies to do it. I note that the FDA has not sent one warning letter to any drug company regarding this issue. FDA has not even sent a letter to Novo Nordis for its Levemir-branded tweet (see “Novo Nordisk’s Branded (Levemir) Tweet is Sleazy Twitter Spam!“).









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




