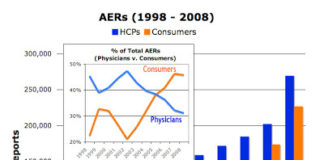
There have been many theories attempting to explain why the FDA is dragging its feet in developing social media guidelines for the drug industry (see, for example, “FDA, DOJ, & Google: Conspiracy Theory, Part 2“). Whatever the reason, financially strapped and under staffed, FDA clearly needs help. To get this help, does the FDA plan to pull in any outside consultants, hire additional experts internally, or approach the drug industry itself to help craft the guidelines?
I learned that FDA Intern, strange visitor from an Ivy League school who came to FDA with powers and ability far beyond those of FDA Commissioner Margaret A. Hamburg or even former FDA commish Andy von Eschenbach, has actually been on the case, attempting to get FDA moving on developing social media guidelines!
FDA Intern! Who can change the course of mighty clinical trials, approve drug ads faster than a speeding bullet, jump through Congressional Subcommittee hoops of fire and ire, and who disguised as Emily Jameson (no relation to Jenna Jameson), mild-mannered intern for a great regulatory agency, fights a never ending battle for fast-track drug approvals, pharmaceutical company user fees, and the FDA way!
Alas, boys and girls, the FDA is not going to get any help from Pfizer’s “Playbook.” For more background & fun summertime reading, enjoy these previous Pharma Marketing Blog posts:
- F**k the FDA! Drug Industry Should Issue Self-Regulatory Social Media Guidelines!
- Citizen Petition Filed by Pharma Likely to Delay Indefinitely the Issuance of FDA Social Media Guidance
- Is This Pfizer’s Social Media “Playbook?”
- Pfizer Asks for New FDA Regulations, Not Guidance, for Social Media
Make sure you are reading the source to get the latest comments.]












![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)