This week marks the six-month anniversary of President Obama signing an Executive Order to help FDA in its efforts to prevent and resolve prescription drug shortages. Following the Executive Order, FDA sent out letters to drug manufacturers asking them to “voluntarily report to FDA if they saw the emerging potential for a drug shortage.”
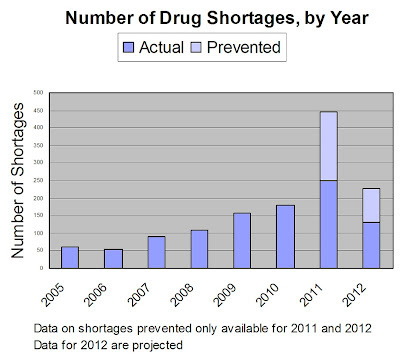
“I am both amazed and delighted to see the progress that’s been made,” said FDA Commissioner Margaret Hamburg in a blog post (here). “Early notification to FDA of potential disruptions in drug supply has made a huge difference in our efforts — and the numbers really tell the story [see chart below].”
“Since reaching out to industry, there has been a six-fold increase in early notifications from manufacturers,” said Hamburg. “Also in that six month timeframe, we have been able to prevent 128 drug shortages, and we’re seeing fewer numbers of shortages occur – 42 new drugs in shortage reported in 2012, compared to 90 new shortages at this time last year. This data is a testament to how FDA exercises flexibility and discretion in much of our work on drug shortages and the importance of strong collaboration and constant communication with industry, health professionals, and patients.”
According to the above chart, FDA projects that there will be only about 130 actual drug shortages reported in 2012 compared to 250 reported in 2011 and that FDA-industry cooperation will have prevented about 100 additional shortages. By FDA estimates, even if it didn’t prevent any shortages, the number of drug shortages in 2012 would be about 230 compared to 250 in 2011. That is, FDA envisions the trend in drug shortages reversing with or without FDA intervention.
But with FDA and industry cooperation, there were only 42 new drugs in shortage reported in 2012, compared to 90 new shortages at this time last year.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




